Hydroxyurea is a newly approved therapeutic agent for the treatment of sickle-cell disease. Hydroxyurea reduces the number of painful crises in sickle-cell patients presumably by increasing the levels of fetal hemoglobin, which has a large solubilizing effect on sickle-cell hemoglobin and reduces polymerization (1). Despite being used to treat a number of cancers for nearly 30 years, the mechanism of how hydroxyurea increases fetal hemoglobin levels remains unclear. In this issue of the JCI, Cokic et al. present results that provide the first explanation of how hydroxyurea increases fetal hemoglobin levels (2). Specifically, they show that fetal hemoglobin increases in response to activation of soluble guanylyl cyclase (sGC) by hydroxyurea-derived NO. The importance of this work manifests itself in at least three ways: 1) the in vitro identification of some of the molecular species involved in fetal hemoglobin induction, 2) a further demonstration of a role for NO in the activity of hydroxyurea, and 3) the application of this work to the development of new NO based treatments for sickle-cell disease.
The work described in this study (2) relies on the authors’ ability to recognize clues from previous basic and clinical research. First, hydroxyurea reacts with enzymes and proteins, particularly heme-containing proteins, to release NO (3–7). Other studies reveal that patients taking hydroxyurea demonstrate increased levels of nitrite, nitrate, and iron nitrosyl hemoglobin, all of which are markers for NO (8–10). Finally, sGC activators increase γ-globin gene expression in both eryth-roleukemic cells and primary human erythroblasts and sGC inhibitors prevent this increase (11). The combination of these results with the ability of NO to stimulate sGC led to the design and execution of the described experiment to determine the effect of nitric oxide donors and hydroxyurea on fetal hemoglobin induction.
Hydroxyurea and NO donors increase γ-globin and cGMP levels
Cokic et al. show that hydroxyurea and two other mechanistically different NO donors increase γ-globin gene expression in K562 erythro-leukemic and human erythroid progenitor cells in a dose-dependent manner. These compounds also increase the γ/β ratio in this system as they have little effect on β-globin gene expression. These cell-based assays, especially the human eryth-roid progenitor model, probably represent the best currently avail-able systems to evaluate human re-sponses to fetal hemoglobin–induc-ing agents. More importantly, hy-droxyurea and S-nitrosocysteine (CysNO), a known NO donor, in-crease fetal hemoglobin levels in human erythroid cells and cGMP in erythroid progenitor cells. sGC in-hibitors block the observed increase in γ-globin gene production. This last group of experiments provides strong evidence for the involvement of sGC in fetal hemoglobin induction and also reveals that hydroxyurea or NO donor-mediated γ-globin gene induction occurs only dur-ing a short period of time during erythroid differentiation.
An NO-mediated pathway for fetal hemoglobin induction
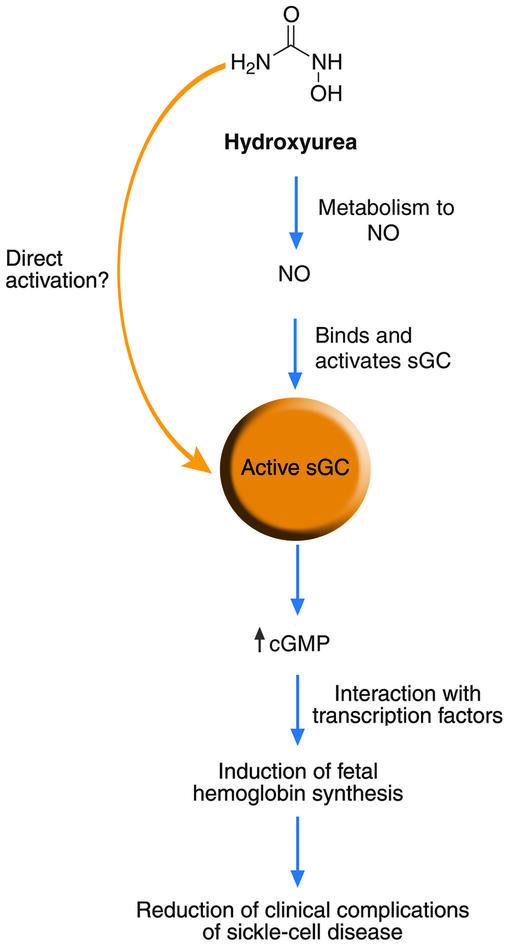
Figure 1 summarizes the current understanding of hydroxyurea-mediated induction of fetal hemoglobin synthesis. Based on the work of Cokic et al. (2) a link between hydroxyurea, NO, sGC, and fetal hemoglobin now exists (Figure 1). Figure 1 also shows where our understanding of this process remains incomplete, particularly: 1) how is hydroxyurea converted to NO in vivo, 2) can hydroxyurea directly activate sGC, and 3) how does an increase in cGMP induce fetal hemoglobin production? Chemically, the conversion of hydroxyurea to NO requires a three-electron oxidation. While a number of heme- and copper-containing proteins react with hydroxyurea to produce NO in vitro through the intermediacy of both radical and nitroso intermediates (3–6), surprisingly little information exists regarding the in vivo oxidative metabolism of hydroxyurea (12). Some work suggests that NO release from hydroxyurea likely occurs during liver metabolism (13), but to date the actual site and oxidant of in vivo hydroxyurea metabolism to NO remains to be identified. Early work shows the ability of urease to catalyze the hydrolysis of hydroxyurea to hydroxylamine, which reacts rapidly with heme proteins to release NO (14). While such a route to NO should be explored, this pathway would be confined to areas of bacterial colonization, such as the gut, as no mammalian ureases exist. The authors present the idea that hydroxyurea may activate sGC through the direct reaction of the ferrous protein with hydroxyurea to produce the iron nitrosyl complex, in analogy to the reaction of hydroxyurea with deoxyhemoglobin, and this idea should also be examined (3). The authors present a number of reasonable but yet-to-be tested ideas involving the stimulation of fetal hemoglobin production by sGC activation (2). Mostly, these ideas elaborate on roles for transcription factors known to be controlled by the NO/cGMP pathway and require further experimentation to delineate the transcription factors responsible for fetal hemoglobin induction. The ability of hydroxyurea to inhibit ribonucleotide reductase and the possibility of this reaction to produce NO should also be further considered (15). At this time, other NO- and sGC-independent pathways effected by hydroxyurea cannot be completely eliminated.
Figure 1.
Mechanism of fetal hemoglobin induction by hydroxyurea. Hydroxyurea is metabolized by an unknown pathway to produce nitric oxide that binds and activates sGC. Alternatively, hydroxyurea may be able to directly activate sGC. Activation of sGC increases production of cGMP, which probably influences some transcription factors leading to increased fetal hemoglobin synthesis.
Finally, these results provide new insight into the development of NO-based therapies for treatment of sickle-cell disease. Inhaled NO and other NO donors, as well as strategies to increase endogenous NO production, should be strongly considered as sickle-cell therapies (16). Recent work by Cokic et al. also shows that the plasma of sickle-cell patients contains cell-free hemoglobin that scavenges nitric oxide (17). The metabolism of hydroxyurea should be rigorously studied to identify the pathways that convert hydroxyurea to NO. The use of cGMP esterase inhibitors, which enhance the effects of NO on sGC, should also be viewed as an alternative approach for sickle-cell disease treatment. The results presented in this paper should excite the audience since they give both clinical and basic scientists a basis for rational therapeutic strategies for sickle-cell disease treatment. Further unraveling hydroxyurea’s metabolism and the mechanism of cGMP-mediated fetal hemoglobin induction should lead to better strategies for sickle-cell disease therapy.
Footnotes
See the related article beginning on page 231.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: soluble guanylyl cyclase (sGC), S-nitrosocysteine (CysNO).
References
- 1.Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin. Hematol. 1997;34:15–21. [PubMed] [Google Scholar]
- 2.Cokic VP, et al. Hydroxyurea induces fetal hemoglobin by the nitric oxide–dependent activation of soluble guanylyl cyclase. J. Clin. Invest. 2003;111:231–239. doi:10.1172/JCI200316672. doi: 10.1172/JCI16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J, et al. Iron nitrosyl hemoglobin formation from the reactions of hemoglobin and hydroxyurea. Biochemistry. 2002;41:2466–2474. doi: 10.1021/bi011470o. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Sommers EM, Kim-Shapiro DB, King SB. Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea. J. Am. Chem. Soc. 2002;124:3473–3480. doi: 10.1021/ja012271v. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, et al. Nitric oxide generation from hydroxyurea via copper-catalyzed peroxidation and implications for pharmacological actions of hydroxyurea. Jpn. J. Cancer Res. 1997;88:1199–1204. doi: 10.1111/j.1349-7006.1997.tb00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacelli R, Taira J, Cook JA, Wink DA, Krishna MC. Hydroxyurea reacts with heme proteins to generate nitric oxide. Lancet. 1996;347:900. doi: 10.1016/s0140-6736(96)91378-1. [DOI] [PubMed] [Google Scholar]
- 7.Stolze K, Nohl H. EPR studies on the oxidation of hydroxyurea to paramagnetic compounds by oxyhemoglobins. Biochem. Pharmacol. 1990;40:799–802. doi: 10.1016/0006-2952(90)90318-f. [DOI] [PubMed] [Google Scholar]
- 8.Glover RE, Ivy ED, Orringer EP, Maeda H, Mason RP. Detection of nitrosyl hemoglobin in venous blood in the treatment of sickle cell anemia with hydroxyurea. Mol. Pharmacology. 1999;55:1006–1010. doi: 10.1124/mol.55.6.1006. [DOI] [PubMed] [Google Scholar]
- 9.Nahavandi M, Wyche MQ, Perlin E, Tavakkoli F, Castro O. Nitric oxide metabolites in sickle cell anemia patients after oral administration of hydroxyurea: hemoglobinopathy. Hematol. 2000;5:335–339. doi: 10.1080/10245332.2000.11746528. [DOI] [PubMed] [Google Scholar]
- 10.Gladwin MT, et al. Nitric oxide donor properties of hydroxyurea in patients with sickle cell disease. Br. J. Hematol. 2002;116:436–444. doi: 10.1046/j.1365-2141.2002.03274.x. [DOI] [PubMed] [Google Scholar]
- 11.Ikuta T, Ausenda S, Cappellini MD. Mechanism for fetal globin gene expression: role of the soluble guanylate cyclase-cGMP-dependent protein kinase pathway. Proc. Natl. Acad. Sci. USA. 2001;98:1847–1852. doi: 10.1073/pnas.041599798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwilt PR, Tracewell WG. Pharmacokinetics and pharmacodynamics of hydroxyurea. Clin. Pharmacokinet. 1998;34:347–356. doi: 10.2165/00003088-199834050-00002. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol. Pharmacology. 1997;52:1081–1086. doi: 10.1124/mol.52.6.1081. [DOI] [PubMed] [Google Scholar]
- 14.Fishbein WN, Carbone PP. Hydroxyurea: mechanism of action. Science. 1963;142:1069–1070. doi: 10.1126/science.142.3595.1069. [DOI] [PubMed] [Google Scholar]
- 15.Yarbro JW. Mechanism of action of hydroxyurea. Semin. Oncol. 1992;19:1–10. [PubMed] [Google Scholar]
- 16.Gladwin MT, Schechter AN. Nitric oxide therapy in sickle cell disease. Semin. Hematol. 2001;38:333–342. doi: 10.1016/s0037-1963(01)90027-7. [DOI] [PubMed] [Google Scholar]
- 17.Reiter CD, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]