Abstract
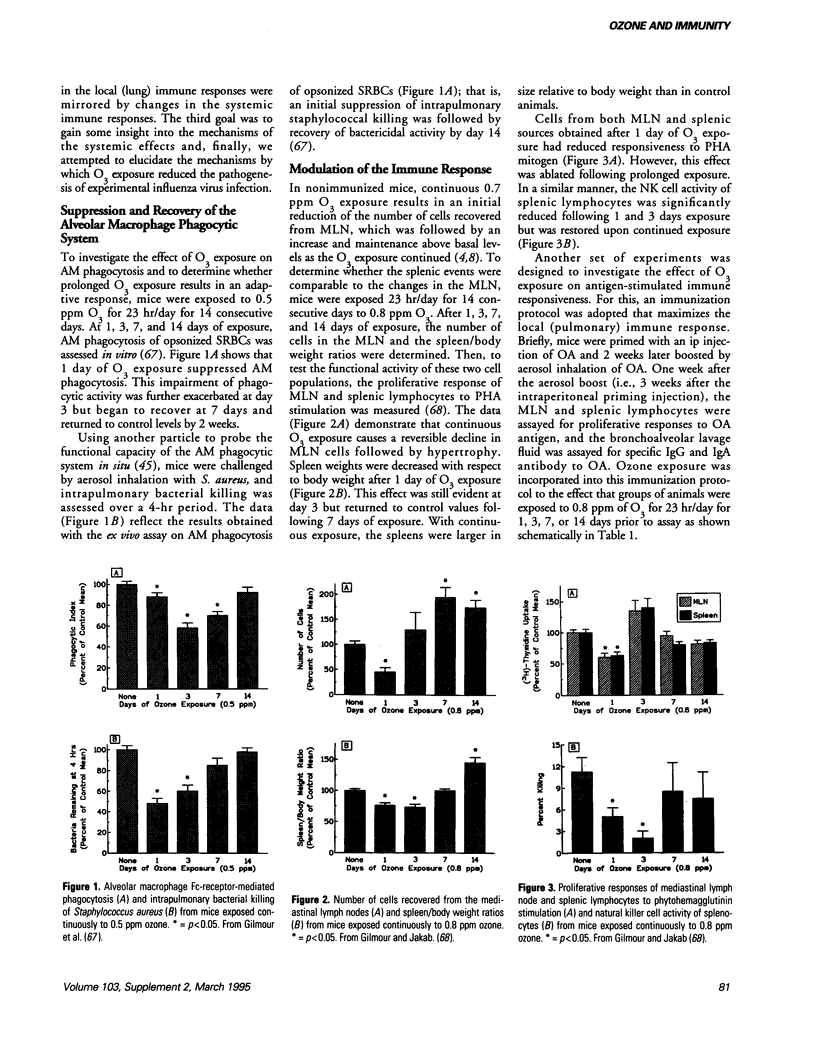
A review of the literature reveals that ozone (O3) exposure can either suppress or enhance immune responsiveness. These disparate effects elicited by O3 exposure depend, in large part, on the experimental design used, the immune parameters examined as well as the animal species studied. Despite the apparent contradictions, a general pattern of response to O3 exposure can be recognized. Most studies indicate that continuous O3 exposure leads to an early (days 0-3) impairment of immune responsiveness followed, with continued exposures, by a form of adaptation to O3 that results in a re-establishment of the immune response. The effects of O3 exposure on the response to antigenic stimulation also depend on the time at which O3 exposure occurred. Whereas O3 exposure prior to immunization is without effect on the response to antigen, O3 exposure subsequent to immunization suppresses the response to antigen. Although most studies have focused on immune responses in the lung, numerous investigators have provided functional and anatomical evidence to support the hypothesis that O3 exposure can have profound effects on systemic immunity.
Full text
PDF



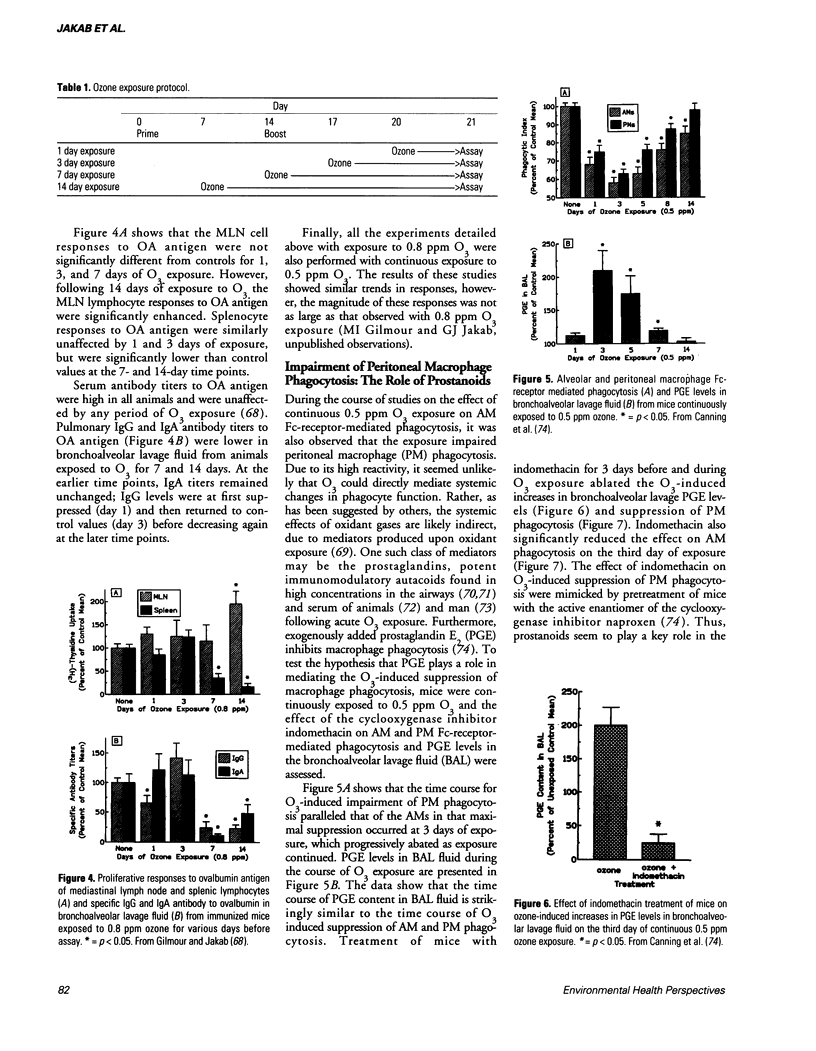





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J. Innate cytotoxicity of CBA mouse spleen cells to Sendai virus-infected L cells. Infect Immun. 1978 Jun;20(3):608–612. doi: 10.1128/iai.20.3.608-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
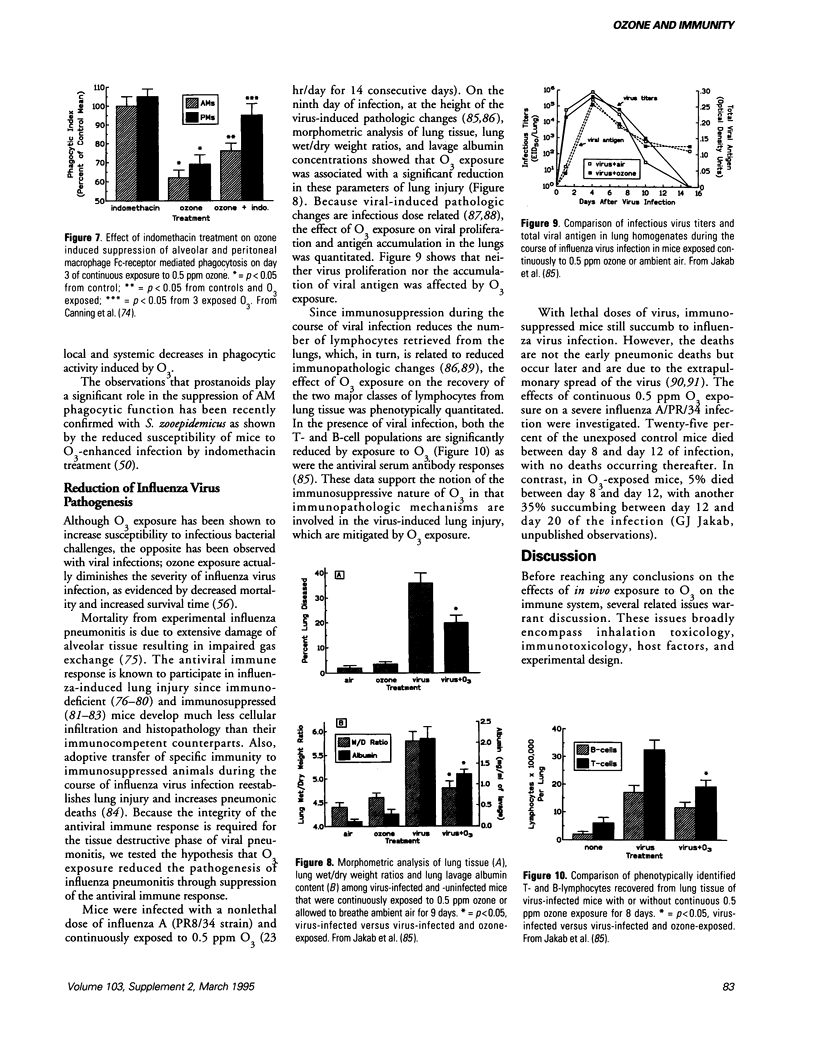
- Atwal O. S., Samagh B. S., Bhatnagar M. K. A possible autoimmune parathyroiditis following ozone inhalation. II. A histopathologic, ultrastructural, and immunofluorescent study. Am J Pathol. 1975 Jul;80(1):53–68. [PMC free article] [PubMed] [Google Scholar]
- BUELL G. C., TOKIWA Y., MUELLER P. K. POTENTIAL CROSSLINKING AGENTS IN LUNG TISSUE. FORMATION AND ISOLATION AFTER IN VIVO EXPOSURE IN OZONE. Arch Environ Health. 1965 Feb;10:213–219. doi: 10.1080/00039896.1965.10663986. [DOI] [PubMed] [Google Scholar]
- Becker S., Jordan R. L., Orlando G. S., Koren H. S. In vitro ozone exposure inhibits mitogen-induced lymphocyte proliferation and IL-2 production. J Toxicol Environ Health. 1989;26(4):469–483. doi: 10.1080/15287398909531270. [DOI] [PubMed] [Google Scholar]
- Becker S., Madden M. C., Newman S. L., Devlin R. B., Koren H. S. Modulation of human alveolar macrophage properties by ozone exposure in vitro. Toxicol Appl Pharmacol. 1991 Sep 15;110(3):403–415. doi: 10.1016/0041-008x(91)90042-d. [DOI] [PubMed] [Google Scholar]
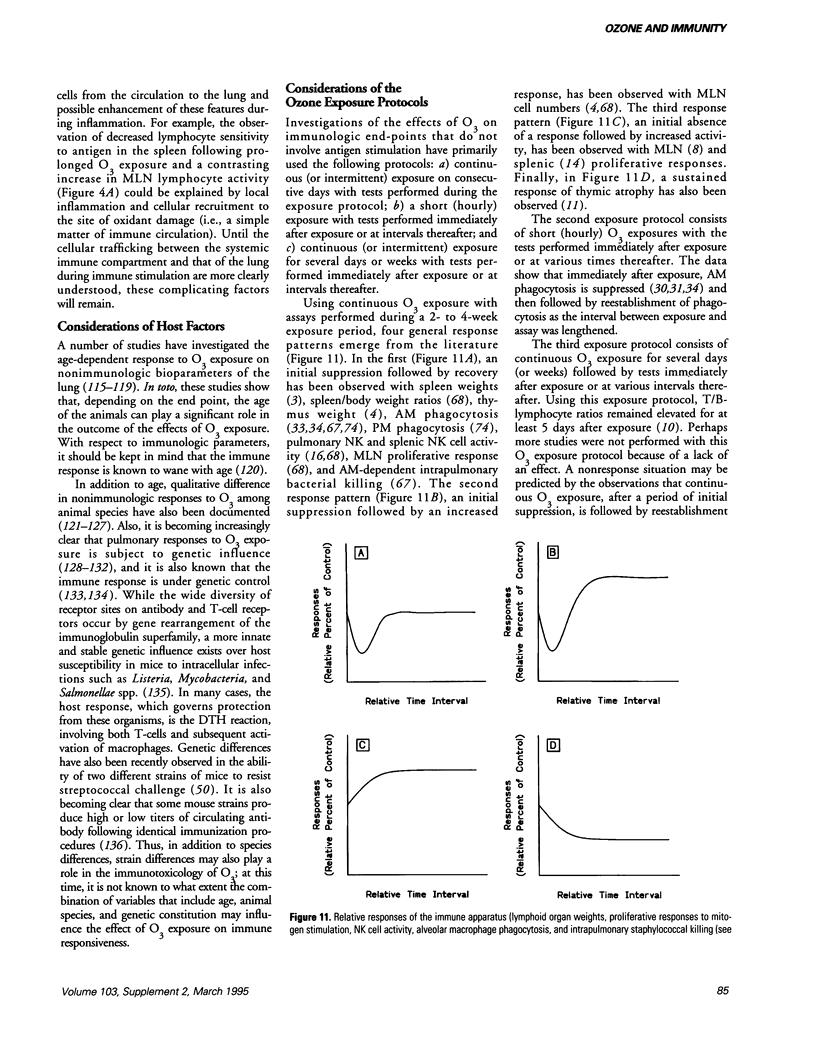
- Becker S., Quay J., Koren H. S. Effect of ozone on immunoglobulin production by human B cells in vitro. J Toxicol Environ Health. 1991 Nov;34(3):353–366. doi: 10.1080/15287399109531573. [DOI] [PubMed] [Google Scholar]
- Berlin B. S., Cochran K. W. Delay of fatal pneumonia in x-irradiated mice inoculated with mouse-adapted influenza virus, PR8 strain. Radiat Res. 1967 Jun;31(2):343–351. [PubMed] [Google Scholar]
- Blandford G. Studies on the immune response and pathogenesis of Sendai virus infection of mice. III. The effects of cyclophosphamide. Immunology. 1975 May;28(5):871–883. [PMC free article] [PubMed] [Google Scholar]
- Bleavins M. R., Dziedzic D. An immunofluorescence study of T and B lymphocytes in ozone-induced pulmonary lesions in the mouse. Toxicol Appl Pharmacol. 1990 Aug;105(1):93–102. doi: 10.1016/0041-008x(90)90361-w. [DOI] [PubMed] [Google Scholar]
- Burleson G. R., Keyes L. L., Stutzman J. D. Immunosuppression of pulmonary natural killer activity by exposure to ozone. Immunopharmacol Immunotoxicol. 1989;11(4):715–735. doi: 10.3109/08923978909005397. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Mold N. G. Increased influenza pneumonia mortality of mice adoptively immunized with node and spleen cells sensitized by inactivated but not live virus. Infect Immun. 1975 May;11(5):908–914. doi: 10.1128/iai.11.5.908-914.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender F. L., Singh B., Cockrell B. Y. The effects in rats and guinea pigs from six months exposures to sulfuric acid mist, ozone, and their combination. J Environ Pathol Toxicol. 1978 Nov-Dec;2(2):485–492. [PubMed] [Google Scholar]
- Christman C. A., Schwartz L. W. Enhanced phagocytosis by alveolar macrophages induced by short-term ozone insult. Environ Res. 1982 Aug;28(2):241–250. doi: 10.1016/0013-9351(82)90127-x. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Harmon R. C. Genetic control of natural cytotoxicity and hybrid resistance. Adv Cancer Res. 1980;31:227–285. doi: 10.1016/s0065-230x(08)60659-4. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., McDonnell W. F., Mann R., Becker S., House D. E., Schreinemachers D., Koren H. S. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol. 1991 Jan;4(1):72–81. doi: 10.1165/ajrcmb/4.1.72. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Vollmuth T. A., Schlesinger R. B. Acute and subchronic ozone inhalation in the rabbit: response of alveolar macrophages. J Toxicol Environ Health. 1987;21(1-2):27–43. doi: 10.1080/15287398709531000. [DOI] [PubMed] [Google Scholar]
- Dziedzic D., White H. J. T-cell activation in pulmonary lymph nodes of mice exposed to ozone. Environ Res. 1986 Dec;41(2):610–622. doi: 10.1016/s0013-9351(86)80155-4. [DOI] [PubMed] [Google Scholar]
- Dziedzic D., White H. J. Thymus and pulmonary lymph node response to acute and subchronic ozone inhalation in the mouse. Environ Res. 1986 Dec;41(2):598–609. doi: 10.1016/s0013-9351(86)80154-2. [DOI] [PubMed] [Google Scholar]
- Dziedzic D., Wright E. S., Sargent N. E. Pulmonary response to ozone: reaction of bronchus-associated lymphoid tissue and lymph node lymphocytes in the rat. Environ Res. 1990 Apr;51(2):194–208. doi: 10.1016/s0013-9351(05)80089-1. [DOI] [PubMed] [Google Scholar]
- Ehrlich R., Findlay J. C., Fenters J. D., Gardner D. E. Health effects of short-term inhalation of nitrogen dioxide and ozone mixtures. Environ Res. 1977 Oct;14(2):223–231. doi: 10.1016/0013-9351(77)90034-2. [DOI] [PubMed] [Google Scholar]
- Ehrlich R., Findlay J. C., Gardner D. E. Effects of repeated exposures to peak concentrations of nitrogen dioxide and ozone on resistance to streptococcal pneumonia. J Toxicol Environ Health. 1979 Jul;5(4):631–642. doi: 10.1080/15287397909529775. [DOI] [PubMed] [Google Scholar]
- Elsayed N. M., Mustafa M. G., Postlethwait E. M. Age-dependent pulmonary response of rats to ozone exposure. J Toxicol Environ Health. 1982 May-Jun;9(5-6):835–848. doi: 10.1080/15287398209530206. [DOI] [PubMed] [Google Scholar]
- Eskew M. L., Scheuchenzuber W. J., Scholz R. W., Reddy C. C., Zarkower A. The effects of ozone inhalation on the immunological response of selenium- and vitamin E-deprived rats. Environ Res. 1986 Aug;40(2):274–284. doi: 10.1016/s0013-9351(86)80103-7. [DOI] [PubMed] [Google Scholar]
- Fairchild G. A. Effects of ozone and sulfur dioxide on virus growth in mice. Arch Environ Health. 1977 Jan-Feb;32(1):28–33. doi: 10.1080/00039896.1977.10667249. [DOI] [PubMed] [Google Scholar]
- Fujimaki H. Impairment of humoral immune responses in mice exposed to nitrogen dioxide and ozone mixtures. Environ Res. 1989 Apr;48(2):211–217. doi: 10.1016/s0013-9351(89)80035-0. [DOI] [PubMed] [Google Scholar]
- Fujimaki H., Shiraishi F., Ashikawa T., Murakami M. Changes in delayed hypersensitivity reaction in mice exposed to O3. Environ Res. 1987 Jun;43(1):186–190. doi: 10.1016/s0013-9351(87)80070-1. [DOI] [PubMed] [Google Scholar]
- Gardner D. E. Use of experimental airborne infections for monitoring altered host defenses. Environ Health Perspect. 1982 Feb;43:99–107. doi: 10.1289/ehp.824399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlinghouse L. E., Jr, Van Hoosier G. L., Jr Studies on adjuvant-induced arthritis, tumor transplantability, and serologic response to bovine serum albumin in Sendai virus-infected rats. Am J Vet Res. 1978 Feb;39(2):297–300. [PubMed] [Google Scholar]
- Gershwin L. J., Osebold J. W., Zee Y. C. Immunoglobulin E-containing cells in mouse lung following allergen inhalation and ozone exposure. Int Arch Allergy Appl Immunol. 1981;65(3):266–277. doi: 10.1159/000232766. [DOI] [PubMed] [Google Scholar]
- Gilmour M. I., Hmieleski R. R., Stafford E. A., Jakab G. J. Suppression and recovery of the alveolar macrophage phagocytic system during continuous exposure to 0.5 ppm ozone. Exp Lung Res. 1991 May-Jun;17(3):547–558. doi: 10.3109/01902149109062864. [DOI] [PubMed] [Google Scholar]
- Gilmour M. I., Park P., Doerfler D., Selgrade M. K. Factors that influence the suppression of pulmonary antibacterial defenses in mice exposed to ozone. Exp Lung Res. 1993 May-Jun;19(3):299–314. doi: 10.3109/01902149309064348. [DOI] [PubMed] [Google Scholar]
- Gilmour M. I., Park P., Selgrade M. K. Ozone-enhanced pulmonary infection with Streptococcus zooepidemicus in mice. The role of alveolar macrophage function and capsular virulence factors. Am Rev Respir Dis. 1993 Mar;147(3):753–760. doi: 10.1164/ajrccm/147.3.753. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Lai L. Y., Ross S. R., Cuzzi-Spada R. Susceptibility of inbred mouse strains to ozone. Arch Environ Health. 1973 Dec;27(6):412–413. doi: 10.1080/00039896.1973.10666416. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Bartlema H. C., van der Ploeg M., van Duijn P., van der Stap J. G., Lippert W. Effect of ozone on lysosomal enzymes of alveolar macrophages engaged in phagocytosis and killing of inhaled Staphylococcus aureus. J Infect Dis. 1978 Sep;138(3):299–311. doi: 10.1093/infdis/138.3.299. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Jordan G. W., MacKenzie M. R., Osebold J. W. Methods for evaluating the toxicological effects of gaseous and particulate contaminants on pulmonary microbial defense systems. Annu Rev Pharmacol Toxicol. 1976;16:447–463. doi: 10.1146/annurev.pa.16.040176.002311. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Tyler W. S., Hoeprich P. D., Eagle C. Ozone and the antibacterial defense mechanisms of the murine lung. Arch Intern Med. 1971 Jun;127(6):1099–1102. [PubMed] [Google Scholar]
- Graham J. A., Gardner D. E., Blommer E. J., House D. E., Ménache M. G., Miller F. J. Influence of exposure patterns of nitrogen dioxide and modifications by ozone on susceptibility to bacterial infectious disease in mice. J Toxicol Environ Health. 1987;21(1-2):113–125. doi: 10.1080/15287398709531006. [DOI] [PubMed] [Google Scholar]
- Gunnison A. F., Finkelstein I., Weideman P., Su W. Y., Sobo M., Schlesinger R. B. Age-dependent effect of ozone on pulmonary eicosanoid metabolism in rabbits and rats. Fundam Appl Toxicol. 1990 Nov;15(4):779–790. doi: 10.1016/0272-0590(90)90194-o. [DOI] [PubMed] [Google Scholar]
- Gunnison A. F., Weideman P. A., Sobo M., Koenig K. L., Chen L. C. Age-dependence of responses to acute ozone exposure in rats. Fundam Appl Toxicol. 1992 Apr;18(3):360–369. doi: 10.1016/0272-0590(92)90134-4. [DOI] [PubMed] [Google Scholar]
- Guth D. J., Warren D. L., Last J. A. Comparative sensitivity of measurements of lung damage made by bronchoalveolar lavage after short-term exposure of rats to ozone. Toxicology. 1986 Aug;40(2):131–143. doi: 10.1016/0300-483x(86)90074-0. [DOI] [PubMed] [Google Scholar]
- Hassett C., Mustafa M. G., Coulson W. F., Elashoff R. M. Splenomegaly in mice following exposure to ambient levels of ozone. Toxicol Lett. 1985 Aug;26(2-3):139–144. doi: 10.1016/0378-4274(85)90158-4. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Slade R., Stead A. G., Graham J. A. Species comparison of acute inhalation toxicity of ozone and phosgene. J Toxicol Environ Health. 1986;19(1):43–53. doi: 10.1080/15287398609530905. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Bassett D. J. Influenza virus infection, ozone exposure, and fibrogenesis. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1307–1315. doi: 10.1164/ajrccm/141.5_Pt_1.1307. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Hmieleski R. R. Reduction of influenza virus pathogenesis by exposure to 0.5 ppm ozone. J Toxicol Environ Health. 1988;23(4):455–472. doi: 10.1080/15287398809531128. [DOI] [PubMed] [Google Scholar]
- Jakab G. J. Suppression of pulmonary antibacterial activity following Sendai virus infection in mice: dependence on virus dose. Arch Virol. 1975;48(4):385–390. doi: 10.1007/BF01317437. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A. Lung defenses against viral and bacterial challenges during immunosuppression with cyclophosphamide in mice. Am Rev Respir Dis. 1981 May;123(5):524–528. doi: 10.1164/arrd.1981.123.5.524. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A., Sannes P. L. Alveolar macrophage ingestion and phagosome-lysosome fusion defect associated with virus pneumonia. Infect Immun. 1980 Mar;27(3):960–968. doi: 10.1128/iai.27.3.960-968.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Long term subclinical effects of parainfluenza (SENDAI) infection on immune cells of aging mice. Proc Soc Exp Biol Med. 1978 Jul;158(3):326–331. doi: 10.3181/00379727-158-40198. [DOI] [PubMed] [Google Scholar]
- Kleeberger S. R., Bassett D. J., Jakab G. J., Levitt R. C. A genetic model for evaluation of susceptibility to ozone-induced inflammation. Am J Physiol. 1990 Jun;258(6 Pt 1):L313–L320. doi: 10.1152/ajplung.1990.258.6.L313. [DOI] [PubMed] [Google Scholar]
- Kleeberger S. R., Kolbe J., Adkinson N. F., Jr, Peters S. P., Spannhake E. W. The role of mediators in the response of the canine peripheral lung to 1 ppm ozone. Am Rev Respir Dis. 1988 Feb;137(2):321–325. doi: 10.1164/ajrccm/137.2.321. [DOI] [PubMed] [Google Scholar]
- Kleeberger S. R., Levitt R. C., Zhang L. Y. Susceptibility to ozone-induced inflammation. II. Separate loci control responses to acute and subacute exposures. Am J Physiol. 1993 Jan;264(1 Pt 1):L21–L26. doi: 10.1152/ajplung.1993.264.1.L21. [DOI] [PubMed] [Google Scholar]
- Li A. F., Richters A. Ambient level ozone effects on subpopulations of thymocytes and spleen T lymphocytes. Arch Environ Health. 1991 Jan-Feb;46(1):57–63. doi: 10.1080/00039896.1991.9937430. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Munson A. E., Thomas P. T., Holsapple M. P., Fenters J. D., White K. L., Jr, Lauer L. D., Germolec D. R., Rosenthal G. J., Dean J. H. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program's guidelines for immunotoxicity evaluation in mice. Fundam Appl Toxicol. 1988 Jan;10(1):2–19. doi: 10.1016/0272-0590(88)90247-3. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Portier C., Pait D. G., White K. L., Jr, Gennings C., Munson A. E., Rosenthal G. J. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam Appl Toxicol. 1992 Feb;18(2):200–210. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- MILLER S., EHRLICH R. Susceptibility to respiratory infections of animals exposed to ozone. I. Susceptibility to Klebsiella pneumoniae. J Infect Dis. 1958 Sep-Oct;103(2):145–149. doi: 10.1093/infdis/103.2.145. [DOI] [PubMed] [Google Scholar]
- Matsumura Y. The effects of ozone, nitrogen dioxide, and sulfur dioxide on the experimentally induced allergic respiratory disorder in guinea pigs. I. The effect on sensitization with albumin through the airway. Am Rev Respir Dis. 1970 Sep;102(3):430–437. doi: 10.1164/arrd.1970.102.3.430. [DOI] [PubMed] [Google Scholar]
- Matsumura Y. The effects of ozone, nitrogen dioxide, and sulfur dioxide on the experimentally induced allergic respiratory disorder in guinea pigs. II. The effects of ozone on the absorption and the retention of antigen in the lung. Am Rev Respir Dis. 1970 Sep;102(3):438–443. doi: 10.1164/arrd.1970.102.3.438. [DOI] [PubMed] [Google Scholar]
- Miller P. D., Ainsworth D., Lam H. F., Amdur M. O. Indomethacin and cromolyn sodium alter ozone-induced changes in lung function and plasma eicosanoid concentrations in guinea pigs. Toxicol Appl Pharmacol. 1988 Apr;93(2):175–186. doi: 10.1016/0041-008x(88)90118-4. [DOI] [PubMed] [Google Scholar]
- Montgomery M. R., Raska-Emery P., Balis J. U. Age-related difference in pulmonary response to ozone. Biochim Biophys Acta. 1987 Feb 11;890(2):271–274. doi: 10.1016/0005-2728(87)90028-4. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., Elsayed N. M., Quinn C. L., Postlethwait E. M., Gardner D. E., Graham J. A. Comparison of pulmonary biochemical effects of low-level ozone exposure on mice and rats. J Toxicol Environ Health. 1982 May-Jun;9(5-6):857–865. doi: 10.1080/15287398209530208. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Kelley G. W., Underdahl N. R. The effect of varied inoculums on the distribution and progression of Influenza virus (S-15) in lungs of mice. Am J Vet Res. 1965 Jul;26(113):984–990. [PubMed] [Google Scholar]
- Oosting R. S., van Golde L. M., Verhoef J., Van Bree L. Species differences in impairment and recovery of alveolar macrophage functions following single and repeated ozone exposures. Toxicol Appl Pharmacol. 1991 Aug;110(1):170–178. doi: 10.1016/0041-008x(91)90299-t. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., Gershwin L. J., Zee Y. C. Studies on the enhancement of allergic lung sensitization by inhalation of ozone and sulfuric acid aerosol. J Environ Pathol Toxicol. 1980 Jun-Jul;3(5-6):221–234. [PubMed] [Google Scholar]
- Osebold J. W., Owens S. L., Zee Y. C., Dotson W. M., LaBarre D. D. Immunological alterations in the lungs of mice following ozone exposure: changes in immunoglobulin levels and antibody-containing cells. Arch Environ Health. 1979 Jul-Aug;34(4):258–265. doi: 10.1080/00039896.1979.10667411. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., Zee Y. C., Gershwin L. J. Enhancement of allergic lung sensitization in mice by ozone inhalation. Proc Soc Exp Biol Med. 1988 Jul;188(3):259–264. doi: 10.3181/00379727-188-42733. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Fujimaki H., Imai T., Honda Y., Watanabe N. Suppression of IgE antibody production after exposure to ozone in mice. Int Arch Allergy Appl Immunol. 1985;76(1):16–19. doi: 10.1159/000233654. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Whiteman M. D., Richter C. B. Susceptibility of inbred and outbred mouse strains to Sendai virus and prevalence of infection in laboratory rodents. Infect Immun. 1978 Jan;19(1):123–130. doi: 10.1128/iai.19.1.123-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. L., Harder S., Rummo N., House D. Effect of ozone on leukocyte function in exposed human subjects. Environ Res. 1978 Jun;15(3):485–493. doi: 10.1016/0013-9351(78)90127-5. [DOI] [PubMed] [Google Scholar]
- SCHEEL L. D., DOBROGORSKI O. J., MOUNTAIN J. T., SVIRBELY J. L., STOKINGER H. E. Physiologic, biochemical, immunologic and pathologic changes following ozone exposure. J Appl Physiol. 1959 Jan;14(1):67–80. doi: 10.1152/jappl.1959.14.1.67. [DOI] [PubMed] [Google Scholar]
- STOKINGER H. E., SCHEEL L. D. Ozone toxicity. Immunochemical and tolerance-producing aspects. Arch Environ Health. 1962 Mar;4:327–334. doi: 10.1080/00039896.1962.10663161. [DOI] [PubMed] [Google Scholar]
- Sagai M., Arakawa K., Ichinose T., Shimojo N. Biochemical effects on combined gases of nitrogen dioxide and ozone. I. Species differences of lipid peroxides and phospholipids in lungs. Toxicology. 1987 Nov;46(3):251–265. doi: 10.1016/0300-483x(87)90207-1. [DOI] [PubMed] [Google Scholar]
- Sanders V. M., Fuchs B. A., Pruett S. B., Kerkvliet N. I., Kaminski N. E. Symposium on indirect mechanisms of immune modulation. Fundam Appl Toxicol. 1991 Nov;17(4):641–650. doi: 10.1016/0272-0590(91)90174-3. [DOI] [PubMed] [Google Scholar]
- Sasazuki T., Nishimura Y., Muto M., Ohta N. HLA-linked genes controlling immune response and disease susceptibility. Immunol Rev. 1983;70:51–75. doi: 10.1111/j.1600-065x.1983.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Savino A., Peterson M. L., House D., Turner A. G., Jeffries H. E., Baker R. The effect of ozone on human cellular and humoral immunity: characterization of T and B lymphocytes by rosette formation. Environ Res. 1978 Feb;15(1):65–69. doi: 10.1016/0013-9351(78)90079-8. [DOI] [PubMed] [Google Scholar]
- Schelegle E. S., Adams W. C., Giri S. N., Siefkin A. D. Acute ozone exposure increases plasma prostaglandin F2 alpha in ozone-sensitive human subjects. Am Rev Respir Dis. 1989 Jul;140(1):211–216. doi: 10.1164/ajrccm/140.1.211. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. B., Driscoll K. E., Gunnison A. F., Zelikoff J. T. Pulmonary arachidonic acid metabolism following acute exposures to ozone and nitrogen dioxide. J Toxicol Environ Health. 1990 Dec;31(4):275–290. doi: 10.1080/15287399009531456. [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Illing J. W., Starnes D. M., Stead A. G., Ménache M. G., Stevens M. A. Evaluation of effects of ozone exposure on influenza infection in mice using several indicators of susceptibility. Fundam Appl Toxicol. 1988 Jul;11(1):169–180. doi: 10.1016/0272-0590(88)90280-1. [DOI] [PubMed] [Google Scholar]
- Singer S. H., Noguchi P., Kirschstein R. L. Respiratory diseases in cyclophosphamide-treated mice. II. Decreased virulence of PR8 influenza virus. Infect Immun. 1972 Jun;5(6):957–960. doi: 10.1128/iai.5.6.957-960.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E. Genetic regulation of host resistance to bacterial infection. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S823–S832. doi: 10.1093/clinids/5.supplement_4.s823. [DOI] [PubMed] [Google Scholar]
- Stewart G. A., Holt P. G. Immunogenicity and tolerogenicity of a major house dust mite allergen, Der p I from Dermatophagoides pteronyssinus, in mice and rats. Int Arch Allergy Appl Immunol. 1987;83(1):44–51. doi: 10.1159/000234329. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Sullivan J. L., Mayner R. E., Barry D. W., Ennis F. A. Influenza virus infection in nude mice. J Infect Dis. 1976 Jan;133(1):91–94. doi: 10.1093/infdis/133.1.91. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Oya J., Ishida N. Effect of antilymphocyte serum on influenza virus infection in mice. Proc Soc Exp Biol Med. 1974 May;146(1):78–84. doi: 10.3181/00379727-146-38047. [DOI] [PubMed] [Google Scholar]
- Sweet C., Smith H. Pathogenicity of influenza virus. Microbiol Rev. 1980 Jun;44(2):303–330. doi: 10.1128/mr.44.2.303-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Tobita K., Seto J. T., Rott R. Comparison of protective effects of serum antibody on respiratory and systemic infection of Sendai virus in mice. Arch Virol. 1989;107(1-2):85–96. doi: 10.1007/BF01313881. [DOI] [PubMed] [Google Scholar]
- Thienes C. H., Skillen R. G., Hoyt A., Bogen E. Effects of ozone on experimental tuberculosis and on natural pulmonary infections in mice. Am Ind Hyg Assoc J. 1965 May-Jun;26(3):255–260. doi: 10.1080/00028896509342728. [DOI] [PubMed] [Google Scholar]
- Thomas G. B., Fenters J. D., Ehrlich R., Gardner D. E. Effects of exposure to ozone on susceptibility to experimental tuberculosis. Toxicol Lett. 1981 Sep;9(1):11–17. doi: 10.1016/0378-4274(81)90168-5. [DOI] [PubMed] [Google Scholar]
- Van Loveren H., Krajnc E. I., Rombout P. J., Blommaert F. A., Vos J. G. Effects of ozone, hexachlorobenzene, and bis(tri-n-butyltin)oxide on natural killer activity in the rat lung. Toxicol Appl Pharmacol. 1990 Jan;102(1):21–33. doi: 10.1016/0041-008x(90)90080-e. [DOI] [PubMed] [Google Scholar]
- Van Loveren H., Rombout P. J., Wagenaar S. S., Walvoort H. C., Vos J. G. Effects of ozone on the defense to a respiratory Listeria monocytogenes infection in the rat. Suppression of macrophage function and cellular immunity and aggravation of histopathology in lung and liver during infection. Toxicol Appl Pharmacol. 1988 Jul;94(3):374–393. doi: 10.1016/0041-008x(88)90279-7. [DOI] [PubMed] [Google Scholar]
- Warr G. A., Jakab G. J., Hearst J. E. Alterations in lung macrophage immune receptor(s) activity associated with viral pneumonia. J Reticuloendothel Soc. 1979 Oct;26(4):357–366. [PubMed] [Google Scholar]
- Warshauer D., Goldstein E., Hoeprich P. D., Lippert W. Effect of vitamin E and ozone on the pulmonary antibacterial defense mechanisms. J Lab Clin Med. 1974 Feb;83(2):228–240. [PubMed] [Google Scholar]
- Wells M. A., Albrecht P., Ennis F. A. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981 Mar;126(3):1036–1041. [PubMed] [Google Scholar]
- Wenzel D. G., Morgan D. L. In vitro inhibition of alveolar macrophage phagocytosis by ozone: absence of a role for serum or mode of ozone administration. Toxicol Lett. 1983 Aug;18(1-2):57–61. doi: 10.1016/0378-4274(83)90071-1. [DOI] [PubMed] [Google Scholar]
- Wolcott J. A., Zee Y. C., Osebold J. W. Exposure to ozone reduces influenza disease severity and alters distribution of influenza viral antigens in murine lungs. Appl Environ Microbiol. 1982 Sep;44(3):723–731. doi: 10.1128/aem.44.3.723-731.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Cate T. R. Cellular changes in lungs of mice infected with influenza virus: characterization of the cytotoxic responses. Infect Immun. 1978 Nov;22(2):423–429. doi: 10.1128/iai.22.2.423-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Couch R. B., Mackler B. F., Cate T. R., Levy B. M. Effects of low- and high-passage influenza virus infection in normal and nude mice. Infect Immun. 1977 Jan;15(1):221–229. doi: 10.1128/iai.15.1.221-229.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Peavy D. L., Cate T. R. Morphological and cytochemical characterization of cells infiltrating mouse lungs after influenza infection. Infect Immun. 1978 Jul;21(1):140–146. doi: 10.1128/iai.21.1.140-146.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


