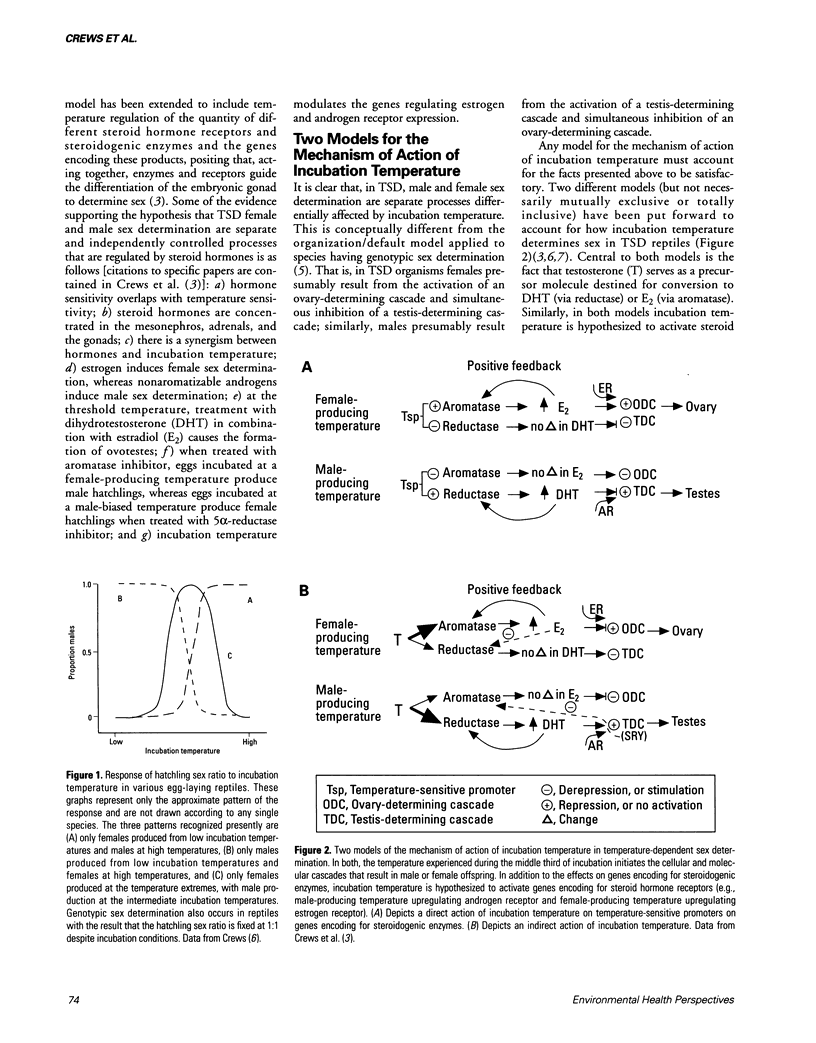
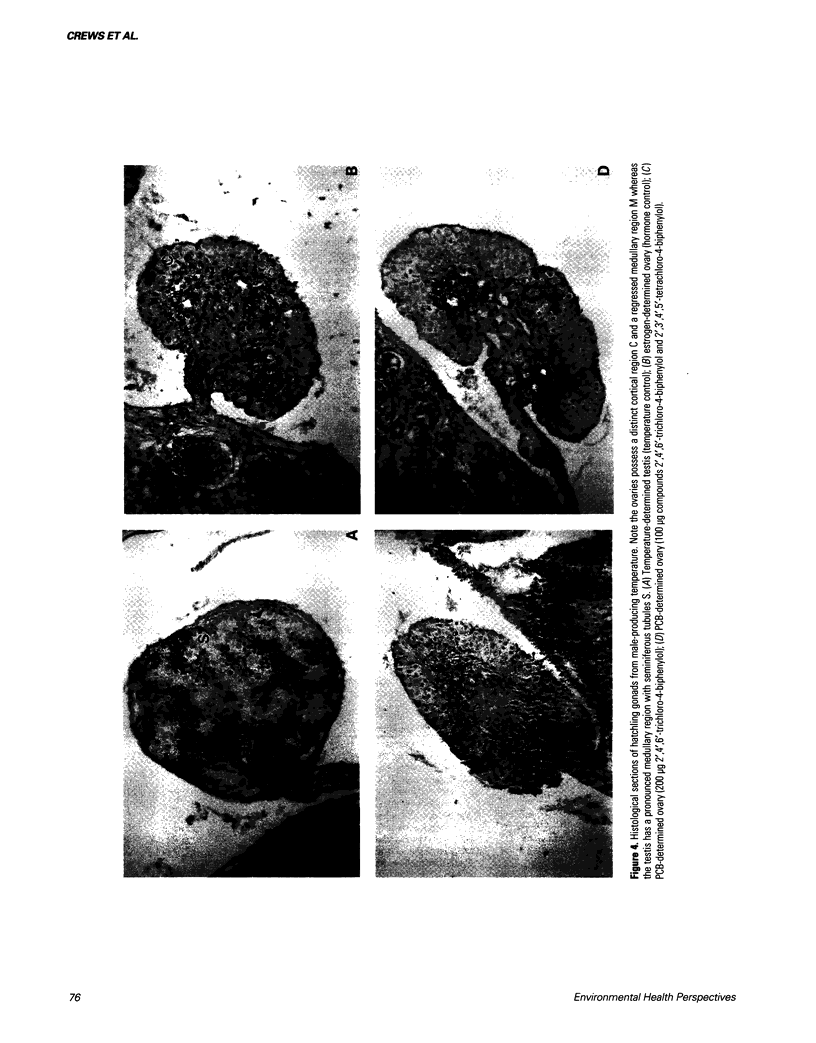
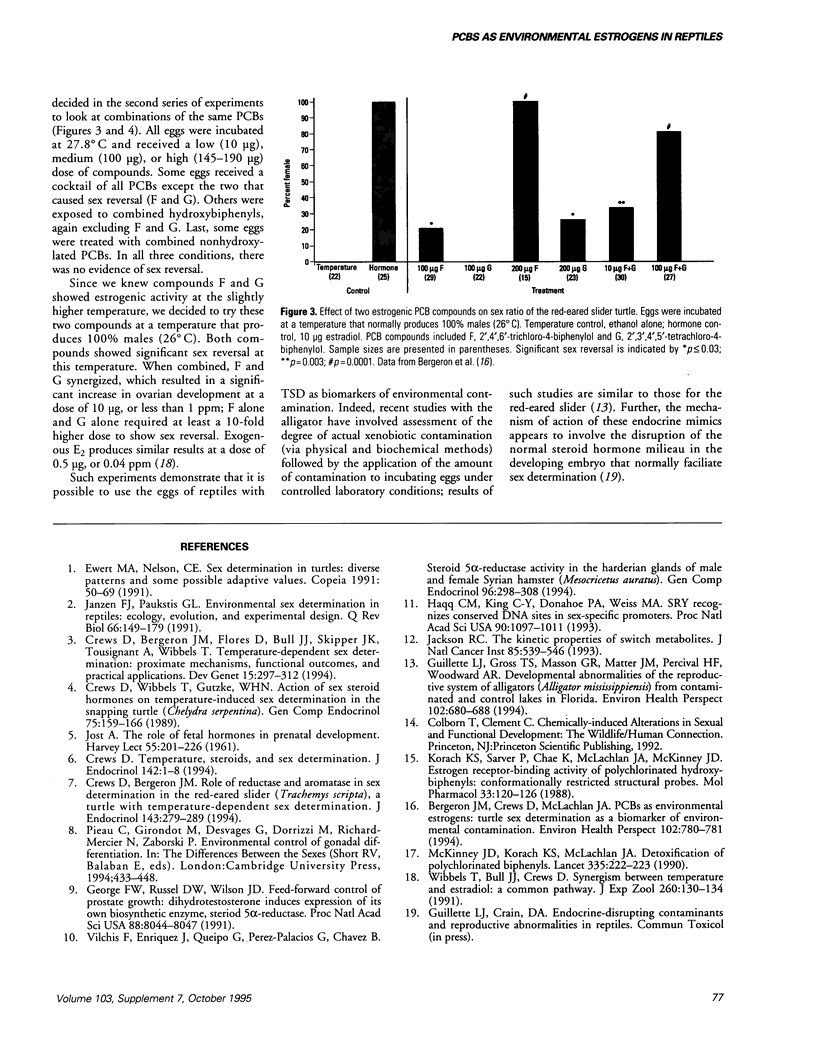
Abstract
In the current model of vertebrate sex determination and sexual differentiation, gonadal sex is fixed at fertilization by specific chromosomes, a process known as genotypic sex determination (GSD). Only after the gonad is formed do hormones begin to exert an influence that modifies specific structures that eventually will differ the sexes. Many egg-laying reptiles do not exhibit GSD but rather depend on the temperature of the incubating egg to determine the gonadal sex of the offspring, a process termed temperature-dependent sex determination (TSD). Research on TSD indicates that sex determination in these species is fundamentally different in at least one way. Gonadal sex is not irrevocably set by the genetic composition inherited at fertilization but depends ultimately on which genes encoding for steroidogenic enzymes and hormone receptors are activated during the midtrimester of embryonic development by temperature. Incubation temperature modifies the activity as well as the temporal and spatial sequence of enzymes and hormone receptors such that sex-specific hormone milieus, created in the urogenital system of the developing embryo, determine gonad type. Estrogen is the physiologic equivalent of incubation temperature and the proximate cue that initiates female sex determination. There is increasing evidence that some polychlorinated biphenyl (PCB) compounds are capable of disrupting reproductive and endocrine function in fish, birds, and mammals, including humans. Reproductive disorders resulting from exposure to these xenobiotic compounds may include reductions in fertility, hatch rate in fish and birds, and viability of offspring, as well as alterations in hormone levels or adult sexual behaviors, all of which have further implications, particularly in wildlife population dynamics. Research on the mechanism through which these compounds may be acting to alter reproductive function indicates estrogenic activity, by which the compounds may be altering sexual differentiation. In TSD turtles, the estrogenic effect of some PCBs reverses gonadal sex in individuals incubating at an otherwise male-producing temperature. Furthermore, certain PCBs are synergistic in their effect at very low concentrations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron J. M., Crews D., McLachlan J. A. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994 Sep;102(9):780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D., Bergeron J. M., Bull J. J., Flores D., Tousignant A., Skipper J. K., Wibbels T. Temperature-dependent sex determination in reptiles: proximate mechanisms, ultimate outcomes, and practical applications. Dev Genet. 1994;15(3):297–312. doi: 10.1002/dvg.1020150310. [DOI] [PubMed] [Google Scholar]
- Crews D., Bergeron J. M. Role of reductase and aromatase in sex determination in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. J Endocrinol. 1994 Nov;143(2):279–289. doi: 10.1677/joe.0.1430279. [DOI] [PubMed] [Google Scholar]
- Crews D. Temperature, steroids and sex determination. J Endocrinol. 1994 Jul;142(1):1–8. doi: 10.1677/joe.0.1420001. [DOI] [PubMed] [Google Scholar]
- Crews D., Wibbels T., Gutzke W. H. Action of sex steroid hormones on temperature-induced sex determination in the snapping turtle (Chelydra serpentina). Gen Comp Endocrinol. 1989 Oct;76(1):159–166. doi: 10.1016/0016-6480(89)90042-7. [DOI] [PubMed] [Google Scholar]
- George F. W., Russell D. W., Wilson J. D. Feed-forward control of prostate growth: dihydrotestosterone induces expression of its own biosynthetic enzyme, steroid 5 alpha-reductase. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8044–8047. doi: 10.1073/pnas.88.18.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqq C. M., King C. Y., Donahoe P. K., Weiss M. A. SRY recognizes conserved DNA sites in sex-specific promoters. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1097–1101. doi: 10.1073/pnas.90.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOST A. The role of fetal hormones in prenatal development. Harvey Lect. 1961;55:201–226. [PubMed] [Google Scholar]
- Jackson R. C. The kinetic properties of switch antimetabolites. J Natl Cancer Inst. 1993 Apr 7;85(7):539–545. doi: 10.1093/jnci/85.7.539. [DOI] [PubMed] [Google Scholar]
- Janzen F. J., Paukstis G. L. Environmental sex determination in reptiles: ecology, evolution, and experimental design. Q Rev Biol. 1991 Jun;66(2):149–179. doi: 10.1086/417143. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Sarver P., Chae K., McLachlan J. A., McKinney J. D. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988 Jan;33(1):120–126. [PubMed] [Google Scholar]
- McKinney J. D., Korach K. S., McLachlan J. A. Detoxification of polychlorinated biphenyls. Lancet. 1990 Jan 27;335(8683):222–223. doi: 10.1016/0140-6736(90)90310-2. [DOI] [PubMed] [Google Scholar]
- Vilchis F., Enriquez J., Queipo G., Pérez-Palacios G., Chávez B. Steroid 5 alpha-reductase activity in the harderian glands of male and female Syrian hamster (Mesocricetus auratus). Gen Comp Endocrinol. 1994 Nov;96(2):298–308. doi: 10.1006/gcen.1994.1185. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Bull J. J., Crews D. Synergism between temperature and estradiol: a common pathway in turtle sex determination? J Exp Zool. 1991 Oct;260(1):130–134. doi: 10.1002/jez.1402600117. [DOI] [PubMed] [Google Scholar]