I loved Upstairs, Downstairs . . . you identify with the downstairs people while vicariously enjoying the life of the upstairs people.
—Alistair Cooke, Host of Masterpiece Theatre
Upstairs, Downstairs was beloved by a generation of Americans who became entranced by this classic story of an upstairs Edwardian family and its downstairs household. The series chronicled the daily repartee and tangled relationships between the upstairs and downstairs contingents, a tale laced with all of the ingredients of human complexity. Despite their diverse perspectives and backgrounds, the lives at 165 Eaton Place became entwined by their core values, conserved traits, and the shared challenges of the day. In terms of human biology, Upstairs, Downstairs was a classic saga of the interplay between genes and environment.
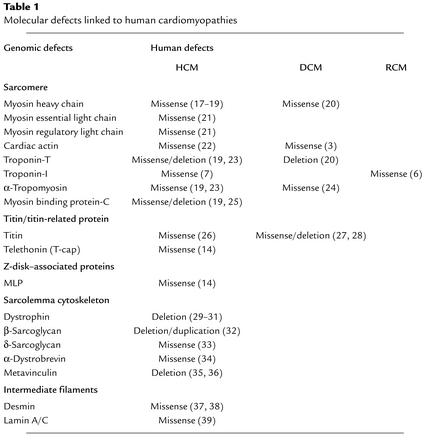
In the family of human cardiomyopathies, another complex story is unfolding, this time around the divergent backgrounds and perspectives of clinical cardiology and molecular genetics. In this cardiological version of Upstairs, Downstairs, the theme is Genotype, Phenotype, and the initial storyline revolves around the precept that the primary determinant of the clinical phenotype is the molecular genotype. The clinical viewpoint has been underpinned by noninvasive analyses that can quantitatively assess differences in chamber volume, wall thickness, hypertrophy, systolic versus diastolic dysfunction, and outflow tract obstruction, leading to three clinical subtypes: hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), and restrictive cardiomyopathy (RCM). The molecular viewpoint has been driven by: 1) the discovery of diverse cardiomyopathic genotypes, resulting in a detailed examination of the differential phenotypic effects of mutations in a myriad of sarcomeric and cytoskeletal genes, 2) cataloguing the effects of missense mutations by the severity of the charge change within a given disease gene, 3) evaluating differences between haploinsufficiency and missense mutations, and 4) correlating these diverse disease genotypes with differences in the severity, time of onset, and diversity of the cardiomyopathy phenotype. The theme of this early script is that there may be a specific set of molecular pathways that account for the distinct cardiac phenotypes of the three major forms of cardiomyopathy. Over a decade ago, the Seidmans made the initial, important discovery that mutations in the β-myosin heavy chain can cause HCM (for review, see ref. 1). Subsequent work by this group and others expanded this concept to include other sarcomeric gene mutations, all of which were linked with the HCM subset of cardiomyopathy. At the same time, studies in genetically engineered mice began to uncover a role for cytoskeletal defects in DCM via studies of mice that harbor a mutation in the cardiac Z disk protein MLP (2), and links between other cytoskeletal proteins and familial forms of human DCM were subsequently established (3) (for review, see refs. 4, 5). In short, the early, neat storyline was that sarcomeric mutations always lead to HCM, while cytoskeletal mutations result in DCM, thereby reflecting specific defects in the hardwiring within cardiac muscle cells that govern these two distinct phenotypes. In short, the phenotypic diversity of familial cardiomyopathies appeared to be primarily driven by the disease genotype. From the perspective of molecular cardiologists, the hunt was on for specific signaling pathways that might differentially connect these genetic lesions with DCM versus HCM. However, recent experimental and clinical studies suggest a more complex genotype-phenotype relationship of cardiomyopathies (Table 1). While there is little doubt that the disease genotype is a critical determinant of the clinical phenotype, perhaps the major protagonists and antagonists of this story have yet to enter the stage.
Table 1.
Molecular defects linked to human cardiomyopathies
Multiple cardiomyopathy phenotypes from identical sarcomeric genotypes: TNNI3 mutations lead to HCM and RCM
In the current issue of the JCI (6) Mogenson et al. reinforce this notion by clearly documenting that a single mutation within the troponin I (TNNI3) gene can lead to either HCM or RCM within the same family. In a series of patients with RCM, a number of independent TNNI3 mutations were uncovered, again suggesting that TNNI3 mutations cannot only lead to HCM, as previously described (7), but also RCM. Previous studies have shown that sarcomeric mutations in the tropomyosin, troponin T, titin, and β-myosin heavy chain gene can lead to either DCM or HCM (Table 1). Taken together, it appears that mutations in a given sarcomeric gene can lead to a spectrum of cardiomyopathic phenotypes, often overlapping between the clinical subsets of DCM, HCM, and RCM. Although there is little doubt that the disease genotype plays a critical role in initiating the cardiomyopathic process, the ultimate clinical phenotype un-doubtedly represents the integrated effect of multiple interacting factors. This view is supported by a host of circumstantial evidence, including the poor penetrance of many cardiomyopathic genotypes, the influence of hemodynamic stress (pressure, volume, etc.) on disease progression (8), the secondary effects of the loss of cardiac myocyte survival and subsequent replacement fibrosis (9), strong modifying effects of calcium cycling (10, 11) and calcium signaling (12), and clear evidence of genetic background effects in gene-targeted mouse models of cardiomyopathy (13).
Resolving cardiomyopathy phenotypes and disease pathways with refined physiological technology: the MLP story
Part of the difficulty in attempting to define the molecular pathways that link the myriad number of sarcomeric and cytoskeletal mutations with specific forms of cardiomyopathy stems from our relatively primitive understanding of the precise physiological phenotype of these and other cardiac muscle diseases. Given the vast diversity of human cardiomyopathic genotypes, as well as the inherent difficulty in assessing the physiological function of cardiac muscle cells from a large number of distinct patients and their families, it is likely that many of the major insights will arise at the interface of mouse models and human disease. For example, recent studies have identified a missense mutation in the muscle LIM domain protein (MLP) gene that is associated with human DCM that has arisen as a result of a founder effect in a Northern European population (14). Parallel studies in MLP-deficient mice indicate that MLP plays a critical role as part of a Z disc-tele-thonin-titin complex that is an essential component of the cardiac muscle stretch sensor (14). The human MLP mutation disrupts this complex, indicating that the pathway that links the disease genotype with the cardiomyopathy phenotype is related to a primary defect in the cardiac muscle stretch sensor pathway (14). Accordingly, it may become possible to develop a more functional approach to the classification of human cardiomyopathies on the basis of a detailed phenotypic analysis of mouse model systems. It will become critical to continue to develop new, high-resolution, high-throughput technology to resolve cardiac muscle physiological phenotypes at the whole organ, intact muscle, and single cell level.
As noted in Table 2, an arsenal of physiological technology has already been developed by multiple laboratories, which can now be coupled with the growing power of mouse genetics and well-characterized gene-targeted model systems. Background effects in various mouse strains have been clearly observed and attempts to map and clone these modifiers are ongoing, a task made easier by the mouse genome project. The generation of hypomorphic alleles that correspond to known human cardiomyopathy genotypes could be especially informative. Conditional mutagenesis will be valuable in assessing whether the onset of cardiomyopathy in the postnatal setting actually reflects subtle, developmental effects of the sarcomeric and cytoskeletal gene mutations on chamber morphogenesis or function and a fleet of CRE recombinase mouse lines have now been well validated to restrict the mutations to specific cardiovascular lineages, i.e., atrial, ventricular, atrioventricular nodal, epicardial, endothelial, and neural crest (for review, see ref. 15). Genetic complementation via germline gene modification or Adeno-associated virus-mediated transcoronary gene transfer should be informative (10, 16), capitalizing on candidate genes uncovered in surrogate systems, i.e., in vitro cardiac myocyte assays, zebrafish, fly, and mouse models.
Table 2.
In vivo and in vitro cardiac physiological phenotyping in mouse models of cardiac diseases
Phenotype, Genotype
Of course, without parallel advances in functional cardiac phenotyping, attempts to use these models to dissect specific molecular pathways for this multifaceted disease are likely to remain superficial. Ironically, the next episodes of the continuing story on the family of cardiomyopathies may rely more on innovative strategies for precision phenotyping, as opposed to simply expanding the number of genetically engineered mouse model systems per se. It is highly likely that a re-analysis of existing gene targeted mouse models of cardiomyopathy with more refined phenotyping will uncover unsuspected physiological mechanisms of direct relevance to cardiac muscle diseases. High-throughput patch clamp arrays, in vivo expression of calcium reporter genes in intracellular micro-domains of living cardiac muscle cells, novel strategies to monitor conduction system function, and new technology to assay cardiac muscle stretch sensor and effector pathways are likely to be featured. Consult your local listing for the sequel to the cardiomyopathy story, Phenotype, Genotype.
Footnotes
See the related article beginning on page 209.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: hypertrophic cardiomyopathy (HCM); dilated cardiomyopathy (DCM); restrictive cardiomyopathy (RCM); troponin I (TNNI3).
References
- 1.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 2.Arber S, et al. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- 3.Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–752. doi: 10.1126/science.280.5364.750. [DOI] [PubMed] [Google Scholar]
- 4.Chien KR. Genomic circuits and the integrative biology of cardiac diseases. Nature. 2000;407:227–232. doi: 10.1038/35025196. [DOI] [PubMed] [Google Scholar]
- 5.Hoshijima M, Chien KR. Mixed signals in heart failure: cancer rules. J. Clin. Invest. 2002;109:849–855. doi:10.1172/JCI200215380. doi: 10.1172/JCI15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mogenson J, et al. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J. Clin. Invest. 2003;111:209–216. doi:10.1172/JCI200316336. doi: 10.1172/JCI16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura A, et al. Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat. Genet. 1997;16:379–382. doi: 10.1038/ng0897-379. [DOI] [PubMed] [Google Scholar]
- 8.Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 9.Hirota H, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 10.Minamisawa S, et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 11.Semsarian C, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J. Clin. Invest. 2002;109:1013–1020. doi:10.1172/JCI200214677. doi: 10.1172/JCI14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang CL, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki M, Carlson KM, Marchuk DA, Rockman HA. Genetic modifier loci affecting survival and cardiac function in murine dilated cardiomyopathy. Circulation. 2002;105:1824–1829. doi: 10.1161/01.cir.0000014926.32463.89. [DOI] [PubMed] [Google Scholar]
- 14.Knoll R, et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–956. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Lozano P, Chien KR. Cre-constructing the heart. Nat. Genet. 2003;33:8–9. doi: 10.1038/ng0103-8. [DOI] [PubMed] [Google Scholar]
- 16.Hoshijima M, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat. Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 17.Tanigawa G, et al. A molecular basis for familial hypertrophic cardiomyopathy: an alpha/beta cardiac myosin heavy chain hybrid gene. Cell. 1990;62:991–998. doi: 10.1016/0092-8674(90)90273-h. [DOI] [PubMed] [Google Scholar]
- 18.Geisterfer-Lowrance AA, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 19.Bonne G, Carrier L, Richard P, Hainque B, Schwartz K. Familial hypertrophic cardiomyopathy: from mutations to functional defects. Circ. Res. 1998;83:580–593. doi: 10.1161/01.res.83.6.580. [DOI] [PubMed] [Google Scholar]
- 20.Kamisago M, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N. Engl. J. Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 21.Poetter K, et al. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat. Genet. 1996;13:63–69. doi: 10.1038/ng0596-63. [DOI] [PubMed] [Google Scholar]
- 22.Mogensen J, et al. Alpha-cardiac actin is a novel disease gene in familial hypertrophic cardiomyopathy. J. Clin. Invest. 1999;103:R39–R43. doi: 10.1172/JCI6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thierfelder L, et al. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 24.Olson TM, Kishimoto NY, Whitby FG, Michels VV. Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. J. Mol. Cell. Cardiol. 2001;33:723–732. doi: 10.1006/jmcc.2000.1339. [DOI] [PubMed] [Google Scholar]
- 25.Bonne G, et al. Cardiac myosin binding protein-C gene splice acceptor site mutation is associated with familial hypertrophic cardiomyopathy. Nat. Genet. 1995;11:438–440. doi: 10.1038/ng1295-438. [DOI] [PubMed] [Google Scholar]
- 26.Satoh M, et al. Structural analysis of the titin gene in hypertrophic cardiomyopathy: identification of a novel disease gene. Biochem. Biophys. Res. Commun. 1999;262:411–417. doi: 10.1006/bbrc.1999.1221. [DOI] [PubMed] [Google Scholar]
- 27.Gerull B, et al. Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy. Nat. Genet. 2002;30:201–204. doi: 10.1038/ng815. [DOI] [PubMed] [Google Scholar]
- 28.Itoh-Satoh M, et al. Titin mutations as the molecular basis for dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2002;291:385–393. doi: 10.1006/bbrc.2002.6448. [DOI] [PubMed] [Google Scholar]
- 29.Towbin JA, et al. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87:1854–1865. doi: 10.1161/01.cir.87.6.1854. [DOI] [PubMed] [Google Scholar]
- 30.Muntoni F, et al. Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N. Engl. J. Med. 1993;329:921–925. doi: 10.1056/NEJM199309233291304. [DOI] [PubMed] [Google Scholar]
- 31.Melacini P, et al. Myocardial involvement is very frequent among patients affected with subclinical Becker’s muscular dystrophy. Circulation. 1996;94:3168–3175. doi: 10.1161/01.cir.94.12.3168. [DOI] [PubMed] [Google Scholar]
- 32.Barresi R, et al. Disruption of heart sarcoglycan complex and severe cardiomyopathy caused by beta sarcoglycan mutations. J. Med. Genet. 2000;37:102–107. doi: 10.1136/jmg.37.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsubata S, et al. Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. J. Clin. Invest. 2000;106:655–662. doi: 10.1172/JCI9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichida F, et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation. 2001;103:1256–1263. doi: 10.1161/01.cir.103.9.1256. [DOI] [PubMed] [Google Scholar]
- 35.Maeda M, Holder E, Lowes B, Valent S, Bies RD. Dilated cardiomyopathy associated with deficiency of the cytoskeletal protein metavinculin. Circulation. 1997;95:17–20. doi: 10.1161/01.cir.95.1.17. [DOI] [PubMed] [Google Scholar]
- 36.Olson TM, et al. Metavinculin mutations alter actin interaction in dilated cardiomyopathy. Circulation. 2002;105:431–437. doi: 10.1161/hc0402.102930. [DOI] [PubMed] [Google Scholar]
- 37.Goldfarb LG, et al. Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat. Genet. 1998;19:402–403. doi: 10.1038/1300. [DOI] [PubMed] [Google Scholar]
- 38.Li D, et al. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation. 1999;100:461–464. doi: 10.1161/01.cir.100.5.461. [DOI] [PubMed] [Google Scholar]
- 39.Fatkin D, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka N, et al. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation. 1996;94:1109–1117. doi: 10.1161/01.cir.94.5.1109. [DOI] [PubMed] [Google Scholar]
- 41.Scherrer-Crosbie M, et al. Determination of right ventricular structure and function in normoxic and hypoxic mice: a transesophageal echocardiographic study. Circulation. 1998;98:1015–1021. doi: 10.1161/01.cir.98.10.1015. [DOI] [PubMed] [Google Scholar]
- 42.Kubota T, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ. Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 43.Williams SP, et al. Dobutamine stress cine-MRI of cardiac function in the hearts of adult cardiomyocyte-specific VEGF knockout mice. J. Magn. Reson. Imaging. 2001;14:374–382. doi: 10.1002/jmri.1197. [DOI] [PubMed] [Google Scholar]
- 44.Milano CA, et al. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 45.McConnell BK, et al. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J. Clin. Invest. 1999;104:1235–1244. doi: 10.1172/JCI7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao YY, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc. Natl. Acad. Sci. USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rockman HA, et al. Molecular and physiological alterations in murine ventricular dysfunction. Proc. Natl. Acad. Sci. USA. 1994;91:2694–2698. doi: 10.1073/pnas.91.7.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pashmforoush M, et al. Adult mice deficient in actinin-associated LIM-domain protein reveal a developmental pathway for right ventricular cardiomyopathy. Nat. Med. 2001;7:591–597. doi: 10.1038/87920. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura T, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 50.Geisterfer-Lowrance AA, et al. A mouse model of familial hypertrophic cardiomyopathy. Science. 1996;272:731–734. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- 51.Zhou YY, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 52.Wussling M, Schenk W, Nilius B. A study of dynamic properties in isolated myocardial cells by the laser diffraction method. J. Mol. Cell Cardiol. 1987;19:897–907. doi: 10.1016/s0022-2828(87)80618-1. [DOI] [PubMed] [Google Scholar]
- 53.Wier WG, Balke CW, Michael JA, Mauban JR. A custom confocal and two-photon digital laser scanning microscope. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H2150–H2156. doi: 10.1152/ajpheart.2000.278.6.H2150. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 2002;3:906–918. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]
- 55.Berul CI, Aronovitz MJ, Wang PJ, Mendelsohn ME. In vivo cardiac electrophysiology studies in the mouse. Circulation. 1996;94:2641–2648. doi: 10.1161/01.cir.94.10.2641. [DOI] [PubMed] [Google Scholar]
- 56.Sah VP, et al. Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J. Clin. Invest. 1999;103:1627–1634. doi: 10.1172/JCI6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kramer K, et al. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J. Pharmacol. Toxicol. Methods. 1993;30:209–215. doi: 10.1016/1056-8719(93)90019-b. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen-Tran VT, et al. A novel genetic pathway for sudden cardiac death via defects in the transition between ventricular and conduction system cell lineages. Cell. 2000;102:671–682. doi: 10.1016/s0092-8674(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 59.Hagendorff A, Schumacher B, Kirchhoff S, Luderitz B, Willecke K. Conduction disturbances and increased atrial vulnerability in Connexin40-deficient mice analyzed by transesophageal stimulation. Circulation. 1999;99:1508–1515. doi: 10.1161/01.cir.99.11.1508. [DOI] [PubMed] [Google Scholar]
- 60.Berul CI, et al. Electrophysiological abnormalities and arrhythmias in alpha MHC mutant familial hypertrophic cardiomyopathy mice. J. Clin. Invest. 1997;99:570–576. doi: 10.1172/JCI119197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berul CI, et al. Familial hypertrophic cardiomyopathy mice display gender differences in electrophysiological abnormalities. J. Interv. Card. Electrophysiol. 1998;2:7–14. doi: 10.1023/a:1009700404218. [DOI] [PubMed] [Google Scholar]
- 62.Kuo HC, et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107:801–813. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 63.Nuss HB, Marban E. Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J. Physiol. 1994;479:265–279. doi: 10.1113/jphysiol.1994.sp020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esposito G, et al. Cellular and functional defects in a mouse model of heart failure. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H3101–H3112. doi: 10.1152/ajpheart.2000.279.6.H3101. [DOI] [PubMed] [Google Scholar]
- 65.Rockman HA, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimoyama M, et al. Calcineurin plays a critical role in pressure overload-induced cardiac hypertrophy. Circulation. 1999;100:2449–2454. doi: 10.1161/01.cir.100.24.2449. [DOI] [PubMed] [Google Scholar]
- 67.Michael LH, et al. Myocardial ischemia and reperfusion: a murine model. Am. J. Physiol. 1995;269:H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 68.Grupp IL, Subramaniam A, Hewett TE, Robbins J, Grupp G. Comparison of normal, hypodynamic, and hyperdynamic mouse hearts using isolated work-performing heart preparations. Am. J. Physiol. 1993;265:H1401–H1410. doi: 10.1152/ajpheart.1993.265.4.H1401. [DOI] [PubMed] [Google Scholar]
- 69.Kudoh S, et al. Mechanical stretch induces hypertrophic responses in cardiac myocytes of angiotensin II type 1a receptor knockout mice. J. Biol. Chem. 1998;273:24037–24043. doi: 10.1074/jbc.273.37.24037. [DOI] [PubMed] [Google Scholar]