Abstract
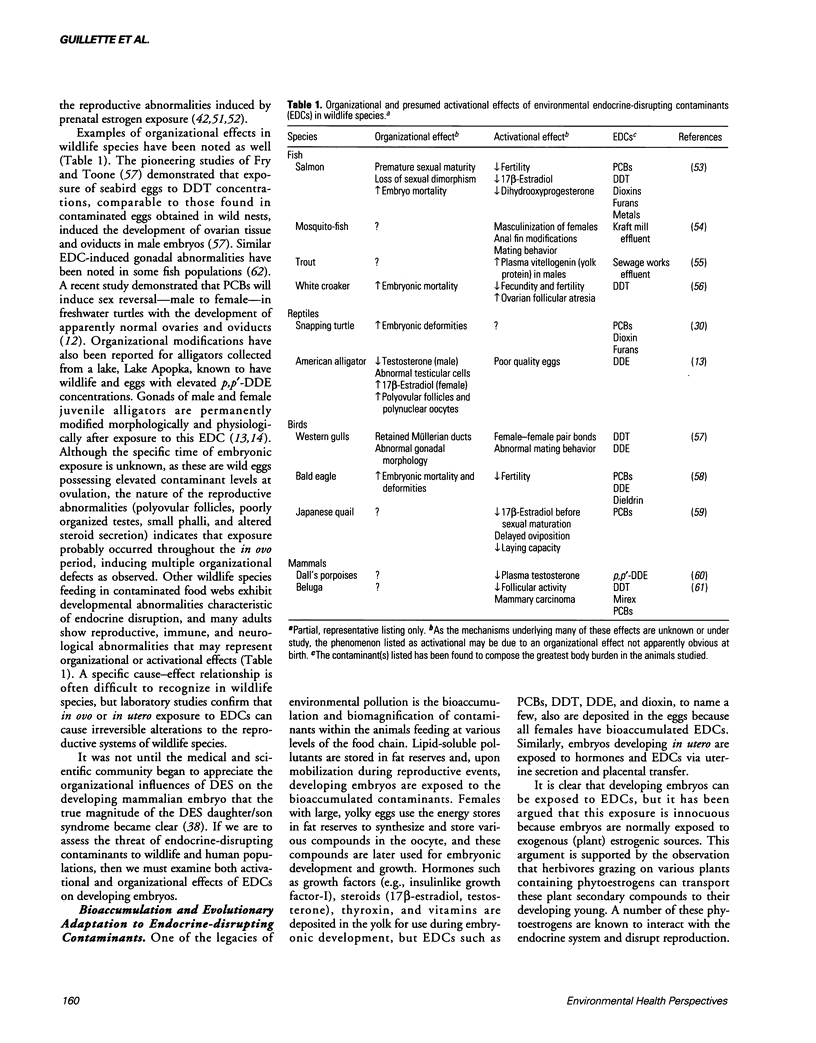
Many environmental contaminants disrupt the vertebrate endocrine system. Although they may be no more sensitive to endocrine-disrupting contaminants (EDCs) than other vertebrates, reptiles are good sentinels of exposure to EDCs due to the lability in their sex determination. This is exemplified by a study of alligators at Lake Apopka, Florida, showing that EDCs have altered the balance of reproductive hormones resulting in reproductive dysfunction. Such alterations may be activationally or organizationally induced. Much research emphasizes the former, but a complete understanding of the influence of EDCs in nature can be generated only after consideration of both activational and organizational alterations. The organizational model suggests that a small quantity of an EDC, administered during a specific period of embryonic development, can permanently modify the organization of the reproductive, immune, and nervous systems. Additionally, this model helps explain evolutionary adaptations to naturally occurring estrogenic compounds, such as phytoestrogens.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H., Bannwart C., Wähälä K., Mäkelä T., Brunow G., Hase T., Arosemena P. J., Kellis J. T., Jr, Vickery L. E. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol. 1993 Feb;44(2):147–153. doi: 10.1016/0960-0760(93)90022-o. [DOI] [PubMed] [Google Scholar]
- Arnold A. P., Breedlove S. M. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985 Dec;19(4):469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Bateman A., Singh A., Kral T., Solomon S. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989 Feb;10(1):92–112. doi: 10.1210/edrv-10-1-92. [DOI] [PubMed] [Google Scholar]
- Berger P. J., Sanders E. H., Gardner P. D., Negus N. C. Phenolic plant compounds functioning as reproductive inhibitors in Microtus montanus. Science. 1977 Feb 11;195(4278):575–577. doi: 10.1126/science.319531. [DOI] [PubMed] [Google Scholar]
- Bergeron J. M., Crews D., McLachlan J. A. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994 Sep;102(9):780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H., Sorkin E. Network of immune-neuroendocrine interactions. Clin Exp Immunol. 1977 Jan;27(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- Bishop C. A., Brooks R. J., Carey J. H., Ng P., Norstrom R. J., Lean D. R. The case for a cause-effect linkage between environmental contamination and development in eggs of the common snapping turtle (Chelydra S.serpentina) from Ontario, Canada. J Toxicol Environ Health. 1991 Aug;33(4):521–547. doi: 10.1080/15287399109531539. [DOI] [PubMed] [Google Scholar]
- Bryan A. M., Stone W. B., Olafsson P. G. Disposition of toxic PCB congeners in snapping turtle eggs: expressed as toxic equivalents of TCDD. Bull Environ Contam Toxicol. 1987 Nov;39(5):791–796. doi: 10.1007/BF01855856. [DOI] [PubMed] [Google Scholar]
- Bull J. J., Gutzke W. H., Crews D. Sex reversal by estradiol in three reptilian orders. Gen Comp Endocrinol. 1988 Jun;70(3):425–428. doi: 10.1016/0016-6480(88)90117-7. [DOI] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D., Bull J. J., Wibbels T. Estrogen and sex reversal in turtles: a dose-dependent phenomenon. Gen Comp Endocrinol. 1991 Mar;81(3):357–364. doi: 10.1016/0016-6480(91)90162-y. [DOI] [PubMed] [Google Scholar]
- Crews D., Wibbels T., Gutzke W. H. Action of sex steroid hormones on temperature-induced sex determination in the snapping turtle (Chelydra serpentina). Gen Comp Endocrinol. 1989 Oct;76(1):159–166. doi: 10.1016/0016-6480(89)90042-7. [DOI] [PubMed] [Google Scholar]
- Deeming D. C., Ferguson M. W. Environmental regulation of sex determination in reptiles. Philos Trans R Soc Lond B Biol Sci. 1988 Dec 1;322(1208):19–39. doi: 10.1098/rstb.1988.0111. [DOI] [PubMed] [Google Scholar]
- Fry D. M., Toone C. K. DDT-induced feminization of gull embryos. Science. 1981 Aug 21;213(4510):922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Girbau M., González-Guerrero P. R., Bassas L., de Pablo F. Insulin receptors and insulin-like growth factor I receptors in embryos from gastrula until organogenesis. Mol Cell Endocrinol. 1992 Dec;90(1):69–75. doi: 10.1016/0303-7207(92)90103-d. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Gross D. A., Rooney A. A., Percival H. F. Gonadal steroidogenesis in vitro from juvenile alligators obtained from contaminated or control lakes. Environ Health Perspect. 1995 May;103 (Suppl 4):31–36. doi: 10.1289/ehp.95103s431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzke W. H., Bull J. J. Steroid hormones reverse sex in turtles. Gen Comp Endocrinol. 1986 Dec;64(3):368–372. doi: 10.1016/0016-6480(86)90070-5. [DOI] [PubMed] [Google Scholar]
- HEATH D. F., VANDEKAR M. TOXICITY AND METABOLISM OF DIELDRIN IN RATS. Br J Ind Med. 1964 Oct;21:269–279. doi: 10.1136/oem.21.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose J. E., Cross J. N., Smith S. G., Diehl D. Reproductive impairment in a fish inhabiting a contaminated coastal environment off Southern California. Environ Pollut. 1989;57(2):139–148. doi: 10.1016/0269-7491(89)90006-7. [DOI] [PubMed] [Google Scholar]
- Hughes C. L., Jr Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ Health Perspect. 1988 Jun;78:171–174. doi: 10.1289/ehp.8878171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen F. J., Paukstis G. L. Environmental sex determination in reptiles: ecology, evolution, and experimental design. Q Rev Biol. 1991 Jun;66(2):149–179. doi: 10.1086/417143. [DOI] [PubMed] [Google Scholar]
- Kalland T. Decreased and disproportionate T-cell population in adult mice after neonatal exposure to diethylstilbestrol. Cell Immunol. 1980 Apr;51(1):55–63. doi: 10.1016/0008-8749(80)90237-3. [DOI] [PubMed] [Google Scholar]
- Kalland T., Forsberg J. G. Delayed hypersensitivity response to oxazolone in neonatally estrogenized mice. Cancer Lett. 1978 Mar;4(3):141–146. doi: 10.1016/s0304-3835(78)94017-x. [DOI] [PubMed] [Google Scholar]
- Kalland T., Strand O., Forsberg J. G. Long-term effects of neonatal estrogen treatment on mitogen responsiveness of mouse spleen lymphocytes. J Natl Cancer Inst. 1979 Aug;63(2):413–421. [PubMed] [Google Scholar]
- Kragh-Hansen U. Molecular aspects of ligand binding to serum albumin. Pharmacol Rev. 1981 Mar;33(1):17–53. [PubMed] [Google Scholar]
- Lance V. A., Bogart M. H. Disruption of ovarian development in alligator embryos treated with an aromatase inhibitor. Gen Comp Endocrinol. 1992 Apr;86(1):59–71. doi: 10.1016/0016-6480(92)90126-5. [DOI] [PubMed] [Google Scholar]
- Leopold A. S., Erwin M., Oh J., Browning B. Phytoestrogens: adverse effects on reproduction in California quail. Science. 1976 Jan 9;191(4222):98–100. doi: 10.1126/science.1246602. [DOI] [PubMed] [Google Scholar]
- Levy J. R., Faber K. A., Ayyash L., Hughes C. L., Jr The effect of prenatal exposure to the phytoestrogen genistein on sexual differentiation in rats. Proc Soc Exp Biol Med. 1995 Jan;208(1):60–66. doi: 10.3181/00379727-208-43832. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Hayes H. T., Korach K., Tucker A. N., Dean J. H., Greenlee W. F., Boorman G. A. Estrogen immunosuppression is regulated through estrogenic responses in the thymus. J Immunol. 1984 Jul;133(1):110–116. [PubMed] [Google Scholar]
- McLachlan J. A. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993 Oct;101(5):386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel C. M. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989 Aug;10(3):232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- Mori T., Mills K. T., Bern H. A. Sensitivity of the vagina and uterus of mice neonatally exposed to estrogen or androgen to postnatal treatment with estrogen or androgen. Proc Soc Exp Biol Med. 1992 Apr;199(4):466–469. doi: 10.3181/00379727-199-43382. [DOI] [PubMed] [Google Scholar]
- Mousavi Y., Adlercreutz H. Genistein is an effective stimulator of sex hormone-binding globulin production in hepatocarcinoma human liver cancer cells and suppresses proliferation of these cells in culture. Steroids. 1993 Jul;58(7):301–304. doi: 10.1016/0039-128x(93)90088-5. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Pentecost B. T., Yamashita S., Lum K., Miller J. V., Nelson P., Blair J., Kong H., Teng C., McLachlan J. A. Female gene expression in the seminal vesicle of mice after prenatal exposure to diethylstilbestrol. Endocrinology. 1989 May;124(5):2568–2576. doi: 10.1210/endo-124-5-2568. [DOI] [PubMed] [Google Scholar]
- Ostrander P. L., Mills K. T., Bern H. A. Long-term responses of the mouse uterus to neonatal diethylstilbestrol treatment and to later sex hormone exposure. J Natl Cancer Inst. 1985 Jan;74(1):121–135. [PubMed] [Google Scholar]
- PHOENIX C. H., GOY R. W., GERALL A. A., YOUNG W. C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959 Sep;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Scavo L., Alemany J., Roth J., de Pablo F. Insulin-like growth factor I activity is stored in the yolk of the avian egg. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1167–1173. doi: 10.1016/0006-291x(89)90796-1. [DOI] [PubMed] [Google Scholar]
- Scavo L., Shuldiner A. R., Serrano J., Dashner R., Roth J., de Pablo F. Genes encoding receptors for insulin and insulin-like growth factor I are expressed in Xenopus oocytes and embryos. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6214–6218. doi: 10.1073/pnas.88.14.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano J., Shuldiner A. R., Roberts C. T., Jr, LeRoith D., de Pablo F. The insulin-like growth factor I (IGF-I) gene is expressed in chick embryos during early organogenesis. Endocrinology. 1990 Sep;127(3):1547–1549. doi: 10.1210/endo-127-3-1547. [DOI] [PubMed] [Google Scholar]
- Sinha N., Lal B., Singh T. P. Pesticides induced changes in circulating thyroid hormones in the freshwater catfish Clarias batrachus. Comp Biochem Physiol C. 1991;100(1-2):107–110. doi: 10.1016/0742-8413(91)90133-e. [DOI] [PubMed] [Google Scholar]
- Tait J. F., Tait S. A. The effect of plasma protein binding on the metabolism of steroid hormones. J Endocrinol. 1991 Dec;131(3):339–357. doi: 10.1677/joe.0.1310339. [DOI] [PubMed] [Google Scholar]
- Takamatsu Y., Iguchi T., Takasugi N. Effects of neonatal exposure to diethylstilbestrol on protein expression by vagina and uterus in mice. In Vivo. 1992 Jan-Feb;6(1):1–8. [PubMed] [Google Scholar]
- Uchima F. D., Vallerga A. K., Firestone G. L., Bern H. A. Effects of early exposure to diethylstilbestrol on cellular protein expression by mouse vaginal epithelium and fibromuscular wall. Proc Soc Exp Biol Med. 1990 Nov;195(2):218–223. doi: 10.3181/00379727-195-43138. [DOI] [PubMed] [Google Scholar]
- Van der Kraak G., Rosenblum P. M., Peter R. E. Growth hormone-dependent potentiation of gonadotropin-stimulated steroid production by ovarian follicles of the goldfish. Gen Comp Endocrinol. 1990 Aug;79(2):233–239. doi: 10.1016/0016-6480(90)90108-x. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Crews D. Putative aromatase inhibitor induces male sex determination in a female unisexual lizard and in a turtle with temperature-dependent sex determination. J Endocrinol. 1994 May;141(2):295–299. doi: 10.1677/joe.0.1410295. [DOI] [PubMed] [Google Scholar]


