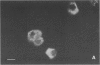
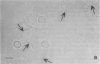
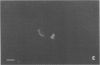
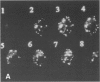
Abstract
Environmental or nutritional estrogenic toxicants are thought to mediate developmental and carcinogenic pathologies. Estrogen receptor (ER) measurements are currently used to predict hormonal responsiveness; therefore all ER subpopulations should be considered. We have been involved in the immunoidentification and characterization of membrane steroid receptors in several systems and have recently shown that binding of estradiol (E2) to a subpopulation of ERs (mER) residing in the plasma membrane of GH3 pituitary tumor cells mediates the rapid release of prolactin (PRL). Here we review these findings and present other important characterizations of these receptors such as trypsin and serum susceptibility, movement in the membrane, confocal localization to the membrane, binding to and function of impeded ligands, and immunoseparation of cells bearing mER. We plan to use this system as a model for both the physiological and pathological nongenomic effects of estrogens and estrogenic xenobiotics. Specifically, it should be useful as an in vitro assay system for the ability of estrogenic xenobiotics to cause rapid PRL release as an example of nongenomic estrogen effects.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Woodland H. R. Changes in the rate of histone synthesis during oocyte maturation and very early development of Xenopus laevis. Dev Biol. 1977 May;57(1):136–149. doi: 10.1016/0012-1606(77)90360-8. [DOI] [PubMed] [Google Scholar]
- Ashburn S. P., Han X., Liehr J. G. Microsomal hydroxylation of 2- and 4-fluoroestradiol to catechol metabolites and their conversion to methyl ethers: catechol estrogens as possible mediators of hormonal carcinogenesis. Mol Pharmacol. 1993 Apr;43(4):534–541. [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein J. D., Lehman M. N., Turcotte J. C., Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992 Jul;131(1):281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Bourguignon G. J. Capping and the cytoskeleton. Int Rev Cytol. 1984;87:195–224. doi: 10.1016/s0074-7696(08)62443-2. [DOI] [PubMed] [Google Scholar]
- Bression D., Brandi A. M., Pagesy P., Le Dafniet M., Martinet M., Brailly S., Michard M., Peillon F. In vitro and in vivo antagonistic regulation by estradiol and progesterone of the rat pituitary domperidone binding sites: correlation with ovarian steroid regulation of the dopaminergic inhibition of prolactin secretion in vitro. Endocrinology. 1985 May;116(5):1905–1911. doi: 10.1210/endo-116-5-1905. [DOI] [PubMed] [Google Scholar]
- Bression D., Michard M., Le Dafniet M., Pagesy P., Peillon F. Evidence for a specific estradiol binding site on rat pituitary membranes. Endocrinology. 1986 Sep;119(3):1048–1051. doi: 10.1210/endo-119-3-1048. [DOI] [PubMed] [Google Scholar]
- Cadena D. L., Gill G. N. Receptor tyrosine kinases. FASEB J. 1992 Mar;6(6):2332–2337. doi: 10.1096/fasebj.6.6.1312047. [DOI] [PubMed] [Google Scholar]
- Caron-Leslie L. M., Schwartzman R. A., Gaido M. L., Compton M. M., Cidlowski J. A. Identification and characterization of glucocorticoid-regulated nuclease(s) in lymphoid cells undergoing apoptosis. J Steroid Biochem Mol Biol. 1991;40(4-6):661–671. doi: 10.1016/0960-0760(91)90288-g. [DOI] [PubMed] [Google Scholar]
- Chen Y. Z., Hua S. Y., Wang C. A., Wu L. G., Gu Q., Xing B. R. An electrophysiological study on the membrane receptor-mediated action of glucocorticoids in mammalian neurons. Neuroendocrinology. 1991;53 (Suppl 1):25–30. doi: 10.1159/000125791. [DOI] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen D. E., Ramirez V. D. Progesterone effects upon dopamine release from the corpus striatum of female rats. II. Evidence for a membrane site of action and the role of albumin. Brain Res. 1989 Jan 9;476(2):338–344. doi: 10.1016/0006-8993(89)91255-9. [DOI] [PubMed] [Google Scholar]
- Dufy B., Partouche C., Poulain D., Dufy-Barbe L., Vincent J. D. Effects of estrogen on the electrical activity of identified and unidentified hypothalamic units. Neuroendocrinology. 1976;22(1):38–47. doi: 10.1159/000122610. [DOI] [PubMed] [Google Scholar]
- Dufy B., Vincent J. D., Fleury H., Du Pasquier P., Gourdji D., Tixier-Vidal A. Membrane effects of thyrotropin-releasing hormone and estrogen shown by intracellular recording from pituitary cells. Science. 1979 May 4;204(4392):509–511. doi: 10.1126/science.107590. [DOI] [PubMed] [Google Scholar]
- Gametchu B. Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science. 1987 Apr 24;236(4800):456–461. doi: 10.1126/science.3563523. [DOI] [PubMed] [Google Scholar]
- Gametchu B., Watson C. S., Pasko D. Size and steroid-binding characterization of membrane-associated glucocorticoid receptor in S-49 lymphoma cells. Steroids. 1991 Aug;56(8):402–410. doi: 10.1016/0039-128x(91)90028-t. [DOI] [PubMed] [Google Scholar]
- Gametchu B., Watson C. S., Shih C. C., Dashew B. Studies on the arrangement of glucocorticoid receptors in the plasma membrane of S-49 lymphoma cells. Steroids. 1991 Aug;56(8):411–419. doi: 10.1016/0039-128x(91)90029-u. [DOI] [PubMed] [Google Scholar]
- Gametchu B., Watson C. S., Wu S. Use of receptor antibodies to demonstrate membrane glucocorticoid receptor in cells from human leukemic patients. FASEB J. 1993 Oct;7(13):1283–1292. doi: 10.1096/fasebj.7.13.8405814. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- Kelly M. J., Moss R. L., Dudley C. A., Fawcett C. P. The specificity of the response of preoptic-septal area neurons to estrogen: 17alpha-estradiol versus 17beta-estradiol and the response of extrahypothalamic neurons. Exp Brain Res. 1977 Oct 24;30(1):43–52. doi: 10.1007/BF00237857. [DOI] [PubMed] [Google Scholar]
- Kelly M. J., Moss R. L., Dudley C. A. The effects of microelectrophoretically applied estrogen, cortisol and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Exp Brain Res. 1977 Oct 24;30(1):53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- Koch B., Lutz-Bucher B., Briaud B., Mialhe C. Specific interaction of corticosteroids with binding sites in the plasma membranes of the rat anterior pituitary gland. J Endocrinol. 1978 Nov;79(2):215–222. doi: 10.1677/joe.0.0790215. [DOI] [PubMed] [Google Scholar]
- Lieberherr M., Grosse B., Kachkache M., Balsan S. Cell signaling and estrogens in female rat osteoblasts: a possible involvement of unconventional nonnuclear receptors. J Bone Miner Res. 1993 Nov;8(11):1365–1376. doi: 10.1002/jbmr.5650081111. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Estradiol regulates the transcription of the prolactin gene. J Biol Chem. 1982 Mar 10;257(5):2133–2136. [PubMed] [Google Scholar]
- McLachlan J. A. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993 Oct;101(5):386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley P., Whitfield J. F., Vanderhyden B. C., Tsang B. K., Schwartz J. L. A new, nongenomic estrogen action: the rapid release of intracellular calcium. Endocrinology. 1992 Sep;131(3):1305–1312. doi: 10.1210/endo.131.3.1505465. [DOI] [PubMed] [Google Scholar]
- Nabekura J., Oomura Y., Minami T., Mizuno Y., Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986 Jul 11;233(4760):226–228. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- Orchinik M., Murray T. F., Franklin P. H., Moore F. L. Guanyl nucleotides modulate binding to steroid receptors in neuronal membranes. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3830–3834. doi: 10.1073/pnas.89.9.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchinik M., Murray T. F., Moore F. L. A corticosteroid receptor in neuronal membranes. Science. 1991 Jun 28;252(5014):1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- Parkening T. A., Collins T. J., Smith E. R. A comparative study of prolactin levels in five species of aged female laboratory rodents. Biol Reprod. 1980 Apr;22(3):513–518. doi: 10.1095/biolreprod22.3.513. [DOI] [PubMed] [Google Scholar]
- Patiño R., Thomas P. Characterization of membrane receptor activity for 17 alpha, 20 beta, 21-trihydroxy-4-pregnen-3-one in ovaries of spotted seatrout (Cynoscion nebulosus). Gen Comp Endocrinol. 1990 May;78(2):204–217. doi: 10.1016/0016-6480(90)90007-9. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Partial purification and characterization of oestrogen receptors in subfractions of hepatocyte plasma membranes. Biochem J. 1980 Dec 1;191(3):743–760. doi: 10.1042/bj1910743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977 Jan 6;265(5589):69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Piroli G., Pignataro O., De Nicola A. F. Increased activity of type I regulatory subunit of cyclic adenosine 3',5'-monophosphate-dependent protein kinase in estrogen-induced pituitary tumors. J Natl Cancer Inst. 1992 Oct 21;84(20):1565–1571. doi: 10.1093/jnci/84.20.1565. [DOI] [PubMed] [Google Scholar]
- Sachs B. D., Leipheimer R. E. Rapid effect of testosterone on striated muscle activity in rats. Neuroendocrinology. 1988 Nov;48(5):453–458. doi: 10.1159/000125049. [DOI] [PubMed] [Google Scholar]
- Sadler S. E., Bower M. A., Maller J. L. Studies of a plasma membrane steroid receptor in Xenopus oocytes using the synthetic progestin RU 486. J Steroid Biochem. 1985 Mar;22(3):419–426. doi: 10.1016/0022-4731(85)90448-0. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Husted R. F., Stokes J. B. Steroid hormone stimulation of Na+ transport in A6 cells is mediated via glucocorticoid receptors. Am J Physiol. 1993 Apr;264(4 Pt 1):C875–C884. doi: 10.1152/ajpcell.1993.264.4.C875. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S. Discussion paper: induction by progesterone and a "maturation-promoting factor" of soluble proteins in Xenopus laevis oocytes in vitro. Ann N Y Acad Sci. 1977 Mar 11;286:421–433. doi: 10.1111/j.1749-6632.1977.tb29434.x. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E. The interaction of steroids with Rana pipiens Oocytes in the induction of maturation. Dev Biol. 1971 Jun;25(2):232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Woodward D. J., Chapin J. K. Sex steroids modulate motor-correlated increases in cerebellar discharge. Brain Res. 1989 Jan 9;476(2):307–316. doi: 10.1016/0006-8993(89)91251-1. [DOI] [PubMed] [Google Scholar]
- Tamarappoo B. K., Nam M., Kilberg M. S., Welbourne T. C. Glucocorticoid regulation of splanchnic glutamine, alanine, glutamate, ammonia, and glutathione fluxes. Am J Physiol. 1993 Apr;264(4 Pt 1):E526–E533. doi: 10.1152/ajpendo.1993.264.4.E526. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Xu X., Norfleet A. M., Watson C. S. The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology. 1993 Jan;132(1):426–430. doi: 10.1210/endo.132.1.8419141. [DOI] [PubMed] [Google Scholar]
- Tischkau S. A., Ramirez V. D. A specific membrane binding protein for progesterone in rat brain: sex differences and induction by estrogen. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1285–1289. doi: 10.1073/pnas.90.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle A. C., Sze P. Y. Steroid binding to synaptic plasma membrane: differential binding of glucocorticoids and gonadal steroids. J Steroid Biochem. 1983 Feb;18(2):135–143. doi: 10.1016/0022-4731(83)90079-1. [DOI] [PubMed] [Google Scholar]
- Waterman M. L., Adler S., Nelson C., Greene G. L., Evans R. M., Rosenfeld M. G. A single domain of the estrogen receptor confers deoxyribonucleic acid binding and transcriptional activation of the rat prolactin gene. Mol Endocrinol. 1988 Jan;2(1):14–21. doi: 10.1210/mend-2-1-14. [DOI] [PubMed] [Google Scholar]
- Wong M., Moss R. L. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992 Aug;12(8):3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B. T., Liehr J. G. Inhibition of the catechol-O-methyltransferase-catalyzed O-methylation of 2- and 4-hydroxyestradiol by catecholamine: implications for the mechanism of estrogen-induced carcinogenesis. Arch Biochem Biophys. 1993 Jul;304(1):248–256. doi: 10.1006/abbi.1993.1346. [DOI] [PubMed] [Google Scholar]
- Zyzek E., Dufy-Barbe L., Dufy B., Vincent J. D. Short-term effect of estrogen on release of prolactin by pituitary cells in culture. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1151–1157. doi: 10.1016/s0006-291x(81)80132-5. [DOI] [PubMed] [Google Scholar]
- de Carvalho-Brunet N., Picart R., Tixier-Vidal A. 17 beta-Estradiol regulates prolactin secretion but not cell proliferation of GH3B6 cells in chemically defined medium. Mol Cell Endocrinol. 1985 Jan;39(1):49–60. doi: 10.1016/0303-7207(85)90091-7. [DOI] [PubMed] [Google Scholar]