Abstract
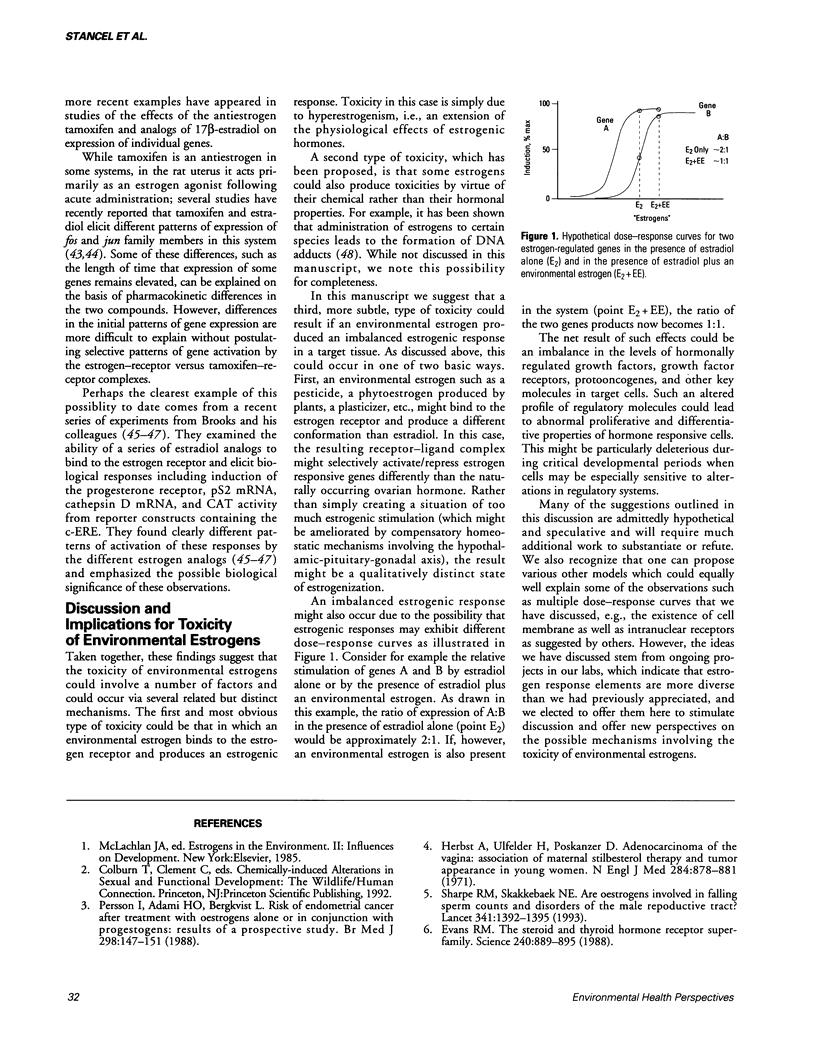
Many naturally occurring and man-made chemicals present in the environment possess estrogenic activity. Examples include plant and fungal products, pesticides, plasticizers, and other agricultural and industrial chemicals. These environmental estrogens as well as endogenous ovarian estrogens are thought to initiate their physiological actions in target tissues largely via interactions with a nuclear receptor system. The resultant estrogen-receptor complex in turn affects transcription via its interactions with nucleotide sequences known as estrogen response elements (EREs) present in the regulatory regions of hormone responsive genes. A "consensus" ERE sequence GGTCAnnnTGACC was originally identified in the vitellogenin genes of birds and amphibians, but it is now clear that most naturally occurring EREs differ from this sequence in one or more bases. We and others have obtained both in vivo and in vitro data suggesting a differential interaction of receptor complexes containing different ligands with the multiple EREs present in mammalian systems. This raises the possibility that the toxicity of environmental estrogens may arise in part from a differential pattern of ERE activation by environmental compounds relative to endogenous ovarian estrogens. The experimental basis for such a paradigm and its toxicological implications are discussed in this paper.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. N., Clark J. H., Peck E. J., Jr The relationship between nuclear receptor-estrogen binding and uterotrophic responses. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1460–1468. doi: 10.1016/0006-291x(72)90878-9. [DOI] [PubMed] [Google Scholar]
- Anderson J. N., Peck E. J., Jr, Clark J. H. Estrogen-induced uterine responses and growth: relationship to receptor estrogen binding by uterine nuclei. Endocrinology. 1975 Jan;96(1):160–167. doi: 10.1210/endo-96-1-160. [DOI] [PubMed] [Google Scholar]
- Anderson J. N., Peck E. J., Jr, Clark J. H. Nuclear receptor-estrogen complex: relationship between concentration and early uterotrophic responses. Endocrinology. 1973 May;92(5):1488–1495. doi: 10.1210/endo-92-5-1488. [DOI] [PubMed] [Google Scholar]
- Berry M., Nunez A. M., Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1218–1222. doi: 10.1073/pnas.86.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Evans M. I., Friedman T. M., O'Malley P. J. Two functional estrogen response elements are located upstream of the major chicken vitellogenin gene. Mol Cell Biol. 1988 Mar;8(3):1123–1131. doi: 10.1128/mcb.8.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Chiappetta C., Kirkland J. L., Loose-Mitchell D. S., Murthy L., Stancel G. M. Estrogen regulates expression of the jun family of protooncogenes in the uterus. J Steroid Biochem Mol Biol. 1992 Feb;41(2):113–123. doi: 10.1016/0960-0760(92)90037-j. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J. Nuclear retention of receptor-oestrogen complex and nuclear acceptor sites. Nature. 1976 Apr 15;260(5552):635–637. doi: 10.1038/260635a0. [DOI] [PubMed] [Google Scholar]
- Darwish H., Krisinger J., Furlow J. D., Smith C., Murdoch F. E., DeLuca H. F. An estrogen-responsive element mediates the transcriptional regulation of calbindin D-9K gene in rat uterus. J Biol Chem. 1991 Jan 5;266(1):551–558. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah J. M., Jr, Rao T. S., Mick S. J., Coyne K. E., Iyengar S. N-methyl-D-aspartate treatment increases circulating adrenocorticotropin and luteinizing hormone in the rat. Endocrinology. 1991 Apr;128(4):1875–1880. doi: 10.1210/endo-128-4-1875. [DOI] [PubMed] [Google Scholar]
- Galand P., Mairesse N., Roorijck J., Flandroy L. Differential blockade of estrogen-induced uterine responses by the antiestrogen nafoxidine. J Steroid Biochem. 1983 Sep;19(3):1259–1263. doi: 10.1016/0022-4731(83)90148-6. [DOI] [PubMed] [Google Scholar]
- Gardner R. M., Kirkland J. L., Stancel G. M. Selective blockade of estrogen-induced uterine responses by the antiestrogen nafoxidine. Endocrinology. 1978 Nov;103(5):1583–1589. doi: 10.1210/endo-103-5-1583. [DOI] [PubMed] [Google Scholar]
- Herbst A. L., Ulfelder H., Poskanzer D. C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971 Apr 15;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Hyder S. M., Stancel G. M. In vitro interaction of uterine estrogen receptor with the estrogen response element present in the 3'-flanking region of the murine c-fos protooncogene. J Steroid Biochem Mol Biol. 1994 Jan;48(1):69–79. doi: 10.1016/0960-0760(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Hyder S. M., Stancel G. M. In vitro interaction of uterine estrogen receptor with the estrogen response element present in the 3'-flanking region of the murine c-fos protooncogene. J Steroid Biochem Mol Biol. 1994 Jan;48(1):69–79. doi: 10.1016/0960-0760(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Hyder S. M., Stancel G. M., Loose-Mitchell D. S. Steroid hormone-induced expression of oncogene encoded nuclear proteins. Crit Rev Eukaryot Gene Expr. 1994;4(1):55–116. doi: 10.1615/critreveukargeneexpr.v4.i1.30. [DOI] [PubMed] [Google Scholar]
- Hyder S. M., Stancel G. M., Nawaz Z., McDonnell D. P., Loose-Mitchell D. S. Identification of an estrogen response element in the 3'-flanking region of the murine c-fos protooncogene. J Biol Chem. 1992 Sep 5;267(25):18047–18054. [PubMed] [Google Scholar]
- Kirkland J. L., Murthy L., Stancel G. M. Tamoxifen stimulates expression of the c-fos proto-oncogene in rodent uterus. Mol Pharmacol. 1993 May;43(5):709–714. [PubMed] [Google Scholar]
- Klein-Hitpass L., Ryffel G. U., Heitlinger E., Cato A. C. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988 Jan 25;16(2):647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge C. M., Peale F. V., Jr, Hilf R., Bambara R. A., Zain S. Cooperative estrogen receptor interaction with consensus or variant estrogen responsive elements in vitro. Cancer Res. 1992 Mar 1;52(5):1073–1081. [PubMed] [Google Scholar]
- Korach K. S., Chae K., Gibson M., Curtis S. Estrogen receptor stereochemistry: ligand binding and hormonal responsiveness. Steroids. 1991 May;56(5):263–270. doi: 10.1016/0039-128x(91)90045-w. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Fox-Davies C., Quarmby V. E., Swaisgood M. H. Diethylstilbestrol metabolites and analogs. Biochemical probes for differential stimulation of uterine estrogen responses. J Biol Chem. 1985 Dec 15;260(29):15420–15426. [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Liehr J. G. Genotoxic effects of estrogens. Mutat Res. 1990 May;238(3):269–276. doi: 10.1016/0165-1110(90)90018-7. [DOI] [PubMed] [Google Scholar]
- Liu Y., Teng C. T. Estrogen response module of the mouse lactoferrin gene contains overlapping chicken ovalbumin upstream promoter transcription factor and estrogen receptor-binding elements. Mol Endocrinol. 1992 Mar;6(3):355–364. doi: 10.1210/mend.6.3.1584212. [DOI] [PubMed] [Google Scholar]
- Maurer R. A., Notides A. C. Identification of an estrogen-responsive element from the 5'-flanking region of the rat prolactin gene. Mol Cell Biol. 1987 Dec;7(12):4247–4254. doi: 10.1128/mcb.7.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch F. E., Gorski J. The role of ligand in estrogen receptor regulation of gene expression. Mol Cell Endocrinol. 1991 Jul;78(3):C103–C108. doi: 10.1016/0303-7207(91)90114-8. [DOI] [PubMed] [Google Scholar]
- Nephew K. P., Polek T. C., Akcali K. C., Khan S. A. The antiestrogen tamoxifen induces c-fos and jun-B, but not c-jun or jun-D, protooncogenes in the rat uterus. Endocrinology. 1993 Jul;133(1):419–422. doi: 10.1210/endo.133.1.8319588. [DOI] [PubMed] [Google Scholar]
- Pentecost B. T., Mattheiss L., Dickerman H. W., Kumar S. A. Estrogen regulation of creatine kinase-B in the rat uterus. Mol Endocrinol. 1990 Jul;4(7):1000–1010. doi: 10.1210/mend-4-7-1000. [DOI] [PubMed] [Google Scholar]
- Persson I., Adami H. O., Bergkvist L., Lindgren A., Pettersson B., Hoover R., Schairer C. Risk of endometrial cancer after treatment with oestrogens alone or in conjunction with progestogens: results of a prospective study. BMJ. 1989 Jan 21;298(6667):147–151. doi: 10.1136/bmj.298.6667.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilat M. J., Hafner M. S., Kral L. G., Brooks S. C. Differential induction of pS2 and cathepsin D mRNAs by structurally altered estrogens. Biochemistry. 1993 Jul 13;32(27):7009–7015. doi: 10.1021/bi00078a028. [DOI] [PubMed] [Google Scholar]
- Richard S., Zingg H. H. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990 Apr 15;265(11):6098–6103. [PubMed] [Google Scholar]
- Savouret J. F., Bailly A., Misrahi M., Rauch C., Redeuilh G., Chauchereau A., Milgrom E. Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J. 1991 Jul;10(7):1875–1883. doi: 10.1002/j.1460-2075.1991.tb07713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe J. W., Chapman L., Finch J. T., Rhodes D. The crystal structure of the estrogen receptor DNA-binding domain bound to DNA: how receptors discriminate between their response elements. Cell. 1993 Nov 5;75(3):567–578. doi: 10.1016/0092-8674(93)90390-c. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Rosenzweig B. A. Identification of an estrogen-responsive element in the rat LH beta gene. DNA-estrogen receptor interactions and functional analysis. J Biol Chem. 1991 Sep 15;266(26):17084–17091. [PubMed] [Google Scholar]
- Slater E. P., Redeuihl G., Theis K., Suske G., Beato M. The uteroglobin promoter contains a noncanonical estrogen responsive element. Mol Endocrinol. 1990 Apr;4(4):604–610. doi: 10.1210/mend-4-4-604. [DOI] [PubMed] [Google Scholar]
- Tora L., Gaub M. P., Mader S., Dierich A., Bellard M., Chambon P. Cell-specific activity of a GGTCA half-palindromic oestrogen-responsive element in the chicken ovalbumin gene promoter. EMBO J. 1988 Dec 1;7(12):3771–3778. doi: 10.1002/j.1460-2075.1988.tb03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderKuur J. A., Hafner M. S., Christman J. K., Brooks S. C. Effects of estradiol-17 beta analogues on activation of estrogen response element regulated chloramphenicol acetyltransferase expression. Biochemistry. 1993 Jul 13;32(27):7016–7021. doi: 10.1021/bi00078a029. [DOI] [PubMed] [Google Scholar]
- VanderKuur J. A., Wiese T., Brooks S. C. Influence of estrogen structure on nuclear binding and progesterone receptor induction by the receptor complex. Biochemistry. 1993 Jul 13;32(27):7002–7008. doi: 10.1021/bi00078a027. [DOI] [PubMed] [Google Scholar]
- Wahli W., Martinez E. Superfamily of steroid nuclear receptors: positive and negative regulators of gene expression. FASEB J. 1991 Jun;5(9):2243–2249. doi: 10.1096/fasebj.5.9.1860615. [DOI] [PubMed] [Google Scholar]
- Weisz A., Bresciani F. Estrogen regulation of proto-oncogenes coding for nuclear proteins. Crit Rev Oncog. 1993;4(4):361–388. [PubMed] [Google Scholar]
- Weisz A., Rosales R. Identification of an estrogen response element upstream of the human c-fos gene that binds the estrogen receptor and the AP-1 transcription factor. Nucleic Acids Res. 1990 Sep 11;18(17):5097–5106. doi: 10.1093/nar/18.17.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Peng X. S., Pugliese T. E., Dickerman H. W., Pentecost B. T. Delineation of sites mediating estrogen regulation of the rat creatine kinase B gene. Mol Endocrinol. 1992 Feb;6(2):231–240. doi: 10.1210/mend.6.2.1569966. [DOI] [PubMed] [Google Scholar]


