Oxidation and nitration of proteins, DNA, and lipids are markers of neurodegeneration in postmortem tissues. It is impossible to determine with certainty using postmortem analysis, whether oxidative stress has a primary role in neurodegeneration or is a secondary end-stage epiphenomenon. Growing evidence suggests that the generation of oxidants does not result simply from an accidental disruption of aerobic metabolism, but rather from an active process crucial for the nonspecific immune defenses of the brain. While essential for survival, these processes may be inappropriately activated to cause neurodegeneration. Neurons are highly susceptible to oxidative stress, which can induce both neuronal necrosis and apoptosis. Oxidants may also have more subtle roles in compromising the integrity of the blood-brain barrier and in producing reactive changes in astrocytes that further propagate injury. Moreover, oxidative stress appears to provide a critical link between environmental factors, such as exposure to pesticides, herbicides, and heavy metals, and endogenous and genetic risk factors in the pathogenic mechanisms of neurodegeneration, particularly in Parkinson disease. Here, we discuss some recent insights into the diverse roles and controversies about the role of oxidants in neurodegeneration. A better understanding of the role of oxidants in neurodegeneration still holds a largely unfulfilled potential to reduce the burden of both acute and chronic neurodegeneration.
Oxidative processes in neuronal systems
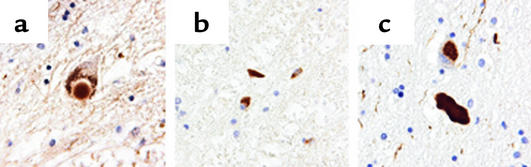
Advances in understanding the chemical nature of oxidative attack on biological molecules have identified many new markers with which to examine postmortem tissues for evidence of oxidative injury. These markers include protein nitrotyrosine, carbonyls in proteins, fatty acid oxidation products, and oxidized DNA bases (1–6). Although biological oxidants have traditionally been viewed as highly reactive and destructive, they can be surprisingly selective and preferentially attack specific sites on macromolecules. For example, specific mAb’s raised to specific sequences from α-synuclein containing nitrotyrosine have revealed that nitrated α-synuclein selectively accumulates in Lewy bodies and protein inclusions in a wide range of pathologies (4) (Figure 1). Tyrosine nitration is one of the earliest markers found in Alzheimer disease brains, in the plaques of multiple sclerosis brains, and in degenerating upper and lower motor neurons in amyotrophic lateral sclerosis (ALS) patients (2, 5, 6). The association of specific oxidative damage with sites of injury in many different types of neurodegeneration suggests a common underlying mechanism. However, these markers could simply reflect secondary epiphenomena rather than having a causal role. A clear delineation of the causal connections cannot be given at present, but a growing body of evidence indicates that oxidants induce distinct pathological consequences that greatly amplify and propagate injury that leads to irreversible degeneration.
Figure 1.
Specific antibodies against nitrated α-synuclein recognize synuclein inclusions. Immunostaining with the mAb nSyn24, a nitrated α-synuclein–specific antibody, reveals staining in nigral Lewy bodies of Parkinson disease and dementia with Lewy bodies (a), glial cytoplasmic inclusions of multiple system atrophy (b), and the Lewy body–like inclusions, neuroaxonal spheroids, and dystrophic neurites of neurodegeneration with brain iron accumulation type 1 (c). The image was provided by John Duda and Benoit Giasson (Center for Neurodegenerative Disease Research, University of Pennsylvania, Philadelphia, USA).
When the generation of oxidants exceeds the rate at which endogenous antioxidant defenses can scavenge oxidants, proteins, lipids, DNA, and other macromolecules become targets for oxidative modification, which leads to deterioration of cellular structural architecture and signaling and ultimately death. Therefore, surviving neurons in human neurological disorders without evidence of biological oxidation could be the cells with the most effective antioxidant capacity. For example, neurons surviving in Huntington disease brains have strongly induced expression of the potent antioxidant en-zyme mitochondrial manganese superoxide dismutase (Mn-SOD) (7). However, oxidative damage of biological targets does not necessarily translate to a pathogenic phenotype, because a multitude of repair processes can be activated to sustain physiological function. In keeping with this, succumbing neurons may be the least proficient in repair capacity. Consistent with this view is the demonstration of decreased repair activity of methionine sulfoxide reductase, an enzymatic activity essential for repair of oxidized methionine residues, in Alzheimer disease brains (8). Methionine sulfoxide reductase (9) or oxidized DNA-repair enzymes may regulate the lifespan of mammals, as mice with mutations in the XPD gene that encodes for a DNA helicase, which is involved in both repair of oxidized DNA lesions and transcription, show evidence of premature aging (10).
Experimentally, the importance of the balance between oxidants and antioxidants has been primarily tested by two approaches in animal and cellular model systems: the genetic elimination of an antioxidant defense mechanism, and the augmentation of antioxidant defenses. In the first paradigm, downregulation of Cu/Zn superoxide dismutase (Cu/Zn-SOD) in mice and cells is associated with increased neuronal injury and death (11, 12). More serious consequences arise from the elimination of the mitochondrial Mn-SOD, which is generally lethal in the neonatal period (13). In addition to causing cardiac failure, the mitochondrial SOD knockout mouse suffers CNS pathology that includes mitochondrial vacuolization and oxidized lipid deposits (13). Mice deficient in glutathione peroxidase, an enzymatic pathway largely responsible for the elimination of hydrogen peroxide and fatty acid peroxides, are more sensitive to ischemia/reperfusion injury and neurotoxins (14). Recently, mice deficient in the α-tocopherol (i.e., vitamin E) transport protein were shown to develop a delayed-onset ataxia and neurodegeneration (15). Conversely, intake of vitamin E has been found to retard the clinical progression of Alzheimer disease (16), and to offer small but significant benefit in ALS patients taking riluzole (17). Recently, treatment with dehydroascorbate has been shown to be protective against stroke (18). Dehydroascorbate was used because it is rapidly taken up into the brain and is reduced to ascorbate. Deficiency in the ascorbate transporter in mice causes lethal cerebral hemorrhages shortly after birth (19).
Metals such as copper and zinc have been shown to accelerate amyloid deposition (20). Redox-active metals such as copper and iron have been implicated in a variety of oxidative processes including Aβ peptide–induced protein oxidation (20) and inactivation of putative antioxidant defenses such as heme oxygenase (21). Chelation of copper by the antibiotic iodochlorhydroxyquin (Clioquinol) has been found to effectively retard amyloid deposition in a mouse model of Alzheimer disease (22).
Genetic and biochemical manipulations to enhance antioxidant defenses provide sound support for the hypothesis that oxidative stress is a critical and common mechanism in neurodegeneration. Overexpression of Cu/Zn-SOD in transgenic mice and rats provides substantial protection against ischemia, cold edema, and neurotoxins and promotes survival of neurons in culture and after transplantation (23–25). Genetically induced increase in expression of Mn-SOD (26) or induction of the enzyme during stress (27) has been shown to protect mitochondria and cells from oxidative stress. Similarly, increased expression of extracellular SOD or glutathione peroxidase, as well as supplementation with SOD mimetics has been found to protect the CNS from a variety of neurotoxins (28, 29). In more simple models, overexpression of SOD and catalase significantly extended the lifespan of flies and worms (30–32). Remarkably, up to 60% of the lifespan of SOD knockout and catalase knockout Drosophila can be restored by expression of SOD in only the motor neurons (32). Overall, new evidence made possible by transgenic approaches implicates antioxidant enzymes and small antioxidants such as vitamins E and C and metal chelation in protecting the CNS.
Sources of oxidants
The generation of oxidants is often attributed to accidents of metabolism, with estimates that up to 1% of all oxygen consumption might be reduced to superoxide or hydrogen peroxide. Certainly, the use oxygen as the terminal electron acceptor poses a serious threat to cells because of the formation of partially reduced oxygen species. Generally, electron flow through oxygen-utilizing processes such as the mitochondrial electron-transport chain, flavoproteins, cytochrome P450, and other oxidases is tightly coupled to avoid partial reduction of oxygen. Any potential interference with the electron transfer, such as the uncoupling of complex I of the mitochondrial electron transport and genetic defects of cytochrome oxidase, may contribute to neuronal degeneration (33, 34).
Oxidants can be produced by essentially all of the cells in the brain. For example, NADPH oxidase, the superoxide-generating enzyme in phagocytes, is expressed not only by microglia but also by astrocytes and neurons (35). However, neither superoxide nor hydrogen peroxide is particularly toxic (36). For toxicity to be observed in most cell culture experiments, the concentration of hydrogen peroxide supplied must be more than the oxygen present in the media. Although hydroxyl radicals are commonly discussed in textbooks as the major toxic oxidant in vivo, their formation by the Haber-Weiss reaction or the Fenton reaction is too slow and too broadly reactive to be particularly toxic (36).
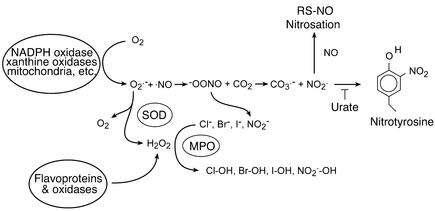
Alternatively, two distinct pathways are used to enhance the toxicity of partially reduced oxygen species. Inflammatory cells greatly enhance the toxicity of hydrogen peroxide by using peroxidases to produce hypochlorous acid (HOCl), better known as bleach, and other hypohalous acids like HOBr and HOI (Figure 2) (37). Cells can greatly increase the toxicity of superoxide by producing nitric oxide. These two radicals react to produce peroxynitrite (ONOO–), by the fastest reaction known in biology and three times faster than previously thought (36). In the presence of carbon dioxide, peroxynitrite readily modifies proteins to form nitrotyrosine. Nitrotyrosine can be also formed by peroxidase oxidation of nitrite, a byproduct of nitric oxide metabolism, and hydrogen peroxide (38). The significance of nitric oxide contribution to neuronal injury is indicated by the use of nitric oxide synthase (NOS) inhibitors and is best documented by the use of mutant mice deficient in the neuronal isoform of NOS (NOS1) (39–43). Mice deficient in NOS1 were found to be resistant to stroke (40), N-methyl-D-aspartate neurotoxicity (41), and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity (24). In addition to NOS1, other studies in human and animal models have also documented the contribution of NOS2, the inducible form of NOS, primarily found in brain glial cells (43, 44). For example, plaques in multiple sclerosis patients showed increased immunoreactivity for NOS2 and nitrotyrosine (6). Nitration has also been associated with compromised integrity of the blood-brain barrier in multiple sclerosis (45). In animal models of multiple sclerosis and stroke, uric acid has proved to be a useful inhibitor of tyrosine nitration and has been shown to protect the blood-brain barrier (45, 46). However, urate does not directly react with peroxynitrite and thus is not strictly a scavenger of peroxynitrite. Nitric oxide derived from NOS2 also contributes to neurotoxicity in ALS mouse models as well as in the MPTP model of Parkinson disease. Blockade of NOS2 and microglia activation by minocycline has been found to be neuroprotective in both Parkinson disease and ALS mouse models, offering hope for a potential therapeutic intervention (47, 48). Activation of microglia leading to peroxynitrite formation has also been linked to Aβ peptide neurotoxicity (49).
Figure 2.
Common reactions of oxygen radicals and nitric oxide in a biological setting. NADPH oxidase and xanthine oxidase transfer a single electron to oxygen to form superoxide anion, while other flavoproteins can transfer two electrons to form hydrogen peroxide directly. SODs scavenge superoxide, which forms additional hydrogen peroxide. Peroxidases use hydrogen peroxide to oxidize a wide range of substrates to produce many different reactive species. Nitric oxide reacts with superoxide to form peroxynitrite. Carbon dioxide reacts catalytically with peroxynitrite to form nitrogen dioxide and carbonate radical. These two radicals nitrate tyrosine, which is blocked by the competitive inhibitor urate. Nitric oxide can also react with these radicals to form nitrosating intermediates that can produce, among other things, S-nitrosothiols. MPO, myeloperoxidase; RS-NO, S-nitrosothiols.
Oxidative and nitrative stress as a unifying mechanism in neurodegeneration
Anatomical and histological studies have established the existence of selective regional vulnerability to neurodegeneration and cell death. For example, the dopaminergic neurons in the substantia nigra are selectively injured in Parkinson disease, whereas motor neurons in the spinal cord are selectively lost in ALS, and loss of cholinergic neurons frequently occurs in the forebrain of individuals with Alzheimer disease. Despite this regional sensitivity, oxidative processes may represent a specific and selective unifying mechanism for neurodegeneration. Several aspects of this working hypothesis are evident in Parkinson disease and ALS.
Parkinson disease.
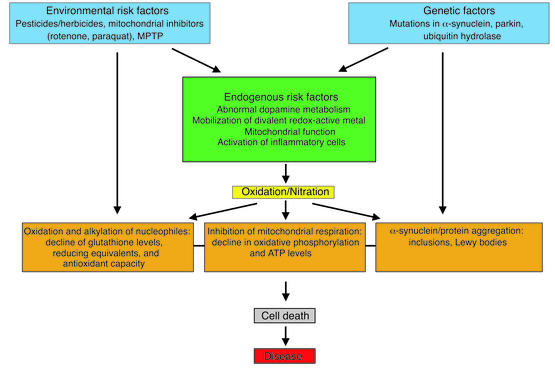
Parkinson disease may serve as an excellent example to discuss the significance of oxidative processes as a central but not an initiating event for the development of clinical disease (Figure 3). Environmental toxins (e.g., paraquat, rotenone, and MPTP) that epidemiological studies have shown to be risk factors are capable of generating reactive intermediates, directly alkylating reduced thiols, inhibiting complex I of the mitochondrial transport chain, inducing α-synuclein aggregation, and activating microglia. Possibly, they may also alter iron or other divalent metal homeostasis as well as dopamine metabolism, permitting an increase in non–vesicle-associated dopamine levels. All these events permit formation of reactive oxygen and nitrogen intermediates that propagate cellular dysfunction, leading to cell death. Support for the working model depicted in Figure 3 comes from rodent and cellular models of Parkinson disease. Chronic administration of the mitochondrial electron-transport inhibitor rotenone was reported to recapitulate features of Parkinson disease in rats, including α-synuclein inclusions (50). Rotenone was also found to induce α-synuclein aggregation in cellular model systems of α-synuclein overexpression (51, 52). Paraquat has been shown to upregulate and induce aggregation of α-synuclein in mice (53) and in α-synuclein–expressing cells (52).
Figure 3.
Proposed model for a central but not initiating role of oxidative processes in the pathogenesis of Parkinson disease. The model is based on published data and incorporates elements of the existing working hypothesis that places oxidative processes at the narrowest point of a funnel through which environmental, genetic, and endogenous risk factors flow to adversely impact cellular function and viability.
The initiating event may also include genetic factors such as mutations of α-synuclein. Mutations of α-synuclein are responsible for familial Parkinson disease (54). However, wild-type α-synuclein appears to represent one of the major building blocks in Lewy bodies as well as other filamentous protein inclusions in sporadic Parkinson disease and several other pathologies (55). With the use of specific antibodies that recognize only the nitrated α-synuclein, it is also apparent that the majority of the Lewy bodies and protein inclusions contain nitrated and possibly oxidized α-synuclein, indicating that oxidative processes may participate in the formation of these inclusions (4) (Figure 1). Based on data from cellular model systems and in vitro biochemical studies, it is likely that oxidative and nitrative processes stabilize the formation of α-synuclein aggregates in a manner that is resistant to proteolysis, thereby allowing the formation of highly insoluble protein aggregates (56–58). The selective nitration of α-synuclein seen in protein inclusions indicates an unexpected biochemical event. Contrary to the belief that oxidants lack ability to select their biological targets, the reactivity is precisely described by the site of formation, as well as the concentration of reactive targets and the second-order rate constants between oxidant and targets. For example, tyrosine nitration is selective largely due to the local environment of certain tyrosine residues in proteins (59). The yield of nitration of these specific residues in selected proteins exceeds the yields typically achieved in proteins without reactive tyrosine residues or free tyrosine in the bulk solution (59). These observa-tions imply that α-synuclein is a primary and specific target for reactive nitrogen intermediates with the four tyrosine residues providing specific substrates for reactivity.
Support for the possibility that oxidative processes are the link between dopamine toxicity and α-synuclein is provided by recent observations that expression of α-synuclein predisposes cells to dopamine toxicity and that oxidants and nitrating agents generated by dopamine promote α-synuclein aggregation (52, 60, 61). The inclusion of dopamine in vesicles may represent a primary pathway for removing the potential toxicity of dopamine (62). In one cellular model, expression of α-synuclein was found to disrupt the process of dopamine transport, increasing the steady-state levels of cytosolic dopamine sufficiently to promote cellular injury and apoptosis (63). The intracellular levels of dopamine may also be regulated by the direct interaction of α-synuclein with tyrosine hydroxylase, the rate-limiting step in dopamine biosynthesis (64). Inhibition of tyrosine hydroxylase activity and a reduction in dopamine levels have been shown to inhibit α-synuclein–induced apoptosis in cultured dopaminergic neurons (61). The toxicity of MPP+ the active metabolite of MPTP, could be directly linked to the rise in intracellular non–vesicle-associated dopamine that leads to the generation of reactive intermediates (65). Furthermore, mice deficient in α-synuclein have been found to have abnormal dopamine release following paired-pulse stimulation and to display a reduction in striatal dopamine, suggesting a possible interplay of α-synuclein with synaptic function related to dopamine metabolism (66). It would be interesting to determine the responses of α-synuclein knockout mice to neurotoxins such as MPTP, paraquat, and mitochondrial inhibitors and to compare and contrast them with the responses of α-synuclein transgenic animals.
Dysregulation of dopamine transport should be considered a critical event in Parkinson disease. Free non–vesicle-associated dopamine is a source of reactive intermediates because of its potential to auto-oxidize to quinone generating reactive intermediates (62). Moreover, quinone addition on reduced sulfhydryls produces covalently linked protein adducts that can inactivate or alter protein function (62). Dopamine adducts with α-synuclein have been recently shown to inhibit formation of α-synuclein fibrils by promoting protofibril formation (67). In vitro studies support a toxic function of protofibrils but not of α-synuclein fibrils, suggesting that oxidative processes may link dopamine and α-synuclein toxicity (67). However, this assertion requires further documentation.
The significance of protein aggregation in neurodegenerative processes is unclear, but, for the most part, the process of protein aggregation and formation of inclusions that contain oxidized and nitrated α-synuclein is not a function of aging but only a phenomenon that coincides with the clinical documentation of Parkinson disease and other related neurodegenerative disorders. The pathological significance of protein aggregation may relate to the effort of the cell to prevent or to attempt to repair the aggregates. It can be envisioned that the cellular ability to repair modified and aggregated protein is diminished due to a significant decline in ATP levels and reducing equivalents. Essentially, a double-hit hypothesis could be evoked, where inhibition of either mitochondrial respiration or protein aggregation alone is not sufficient to drive the cell to its demise but the combination of the two can indeed execute cell death. Overall, oxidative processes may operate in concert with other molecular events such as failure of energy production and respiration, ability to repair oxidized biomolecules and to sustain synaptic contacts and trophic support in the process of neurodegeneration. Therefore it is likely that antioxidant therapies are only partly protective, since they do not alleviate the pressure or burden of sustaining synaptic transmission and metabolic and trophic support that are equally important for cell survival.
ALS.
The difficulties of establishing a causal connection between oxidative stress and neurodegeneration are perhaps best illustrated in ALS. ALS results from the progressive death of motor neurons, leading to rapid muscle degeneration and progressive paralysis. A decade ago, the first 13 of nearly a hundred different mutations were identified in the SOD1 locus encoding the cytosolic Cu/Zn-SOD (see www.alsod.org). Transgenic mice and rats expressing mutant SOD1 develop motor neuron disease, which clearly establishes a toxic gain of function in SOD (68).
These Cu/Zn-SOD mutations would appear to be a smoking gun for oxidative stress causing neurodegeneration. One can find lipid peroxidation and nitrotyrosine in both transgenic mice and human patients (5, 68). Yet, after a decade of research, the role of oxidants remains confusing, with many researchers now favoring protein aggregation over any causal involvement of oxidative stress in ALS (69). The mutations to Cu/Zn-SOD reveal how little we truly understand about the interplay of oxidants and antioxidant defenses like SOD in the brain. The antioxidant defenses against superoxide provided by SOD are formidable. In nontransgenic mice, SOD concentration is about 14 μM (based on monomer size of 16,000 daltons) whereas oxygen concentrations are typically only 30 μM. In the transgenic mice expressing mutant Cu/Zn-SOD, the concentrations of mutant protein are about 80 μM in the “low-expressing” lines or nearly 6% of total protein. Enhancing any malfunction of such an abundant protein can have huge consequences.
We found that even the mutant Cu/Zn-SODs that cause the most rapid forms of ALS can form perfectly functional proteins with normal SOD activity (70). However, the mutant proteins are clearly less stable and more susceptible to losing their zinc and copper atoms. Because zinc is held about 7,000 times less tightly than copper, the mutations favor the accumulation of zinc-deficient Cu/Zn-SOD. The loss of zinc visibly changes the protein from green to blue (70, 71). The copper in zinc-deficient Cu/Zn-SOD is more accessible, and zinc-deficient SOD rapidly oxidizes many intracellular antioxidants, like ascorbate and thiols. The copper in turn transfers these electrons to oxygen to produce superoxide, in effect allowing Cu/Zn-SOD to operate in reverse. With a low concentration of nitric oxide, zinc-deficient Cu/Zn-SOD catalyzes the generation of peroxynitrite while depleting cellular antioxidant defenses (71). Endogenous production of peroxynitrite is sufficient to activate apoptosis in motor neurons by activating caspases (72). Delivering zinc-deficient Cu/Zn-SOD to motor neurons is sufficient to cause motor neurons to die through a peroxynitrite-dependent mechanism (71). The zinc-deficient Cu/Zn-SOD hypothesis offers reasonable explanations for how Cu/Zn-SOD can be involved in ALS. Zinc-deficient wild-type Cu/Zn-SOD is just as toxic as zinc-deficient mutant Cu/Zn-SOD, which explains how wild-type Cu/Zn-SOD could be involved in the vast majority of ALS cases without mutations in Cu/Zn-SOD. The mutations only slightly weaken the affinity for zinc, and thus the potential for forming zinc-deficient Cu/Zn-SOD is increased.
However, some data in transgenic mice appear to argue against the zinc-deficient Cu/Zn-SOD hypothesis. Most recently, knocking out the gene for the copper chaperone for Cu/Zn-SOD (CCS) was found not to affect disease development in transgenic mice overexpressing mutant Cu/Zn-SODs (73). However, 15% of Cu/Zn-SOD activity remained in the CCS knockout mice, showing that the Cu/Zn-SOD could incorporate approximately 12 μM copper from another cellular source possibly generating zinc-deficient Cu/Zn-SOD. The zinc-deficient Cu/Zn-SOD hypothesis can explain the role of Cu/Zn-SOD in promoting the death of motor neurons, but not why the disease is progressive, causing the death of the few hundred thousand neurons that control all voluntary muscle contraction. Recently, peroxynitrite has been shown to provoke a long-lasting reactive phenotype in spinal cord astrocytes, and astrocytes have been shown to cause motor neurons in coculture to undergo apoptosis (74). In contrast, treatment of astrocytes with hydrogen peroxide does not induce apoptosis of cocultured motor neurons. A consistent finding in ALS spinal cord and in transgenic mice is an extremely strong immunoreactivity for nitrotyrosine associated with reactive astrocytes (5). By provoking surrounding astrocytes, a cluster of dying motor neurons may initiate a progressive death of neighboring motor neurons. Although the zinc-deficient Cu/Zn-SOD hypothesis is highly controversial, it illustrates how much more subtle the role of oxidative and nitrative stress can be in neurodegeneration. Rather than causing overt necrotic death in motor neurons, oxidants can kill motor neurons by activating an apoptotic cascade far upstream. Instead of killing astrocytes, some oxidants induce a “proinflammatory” reactive phenotype that causes motor neurons to produce peroxynitrite and thus to activate apoptosis.
Concluding comments and future directions
Currently, there is sufficient documentation to place oxidative and nitrative processes in the center of the pathogenic mechanism that leads to neuronal loss and neurodegeneration. However, despite years of research efforts, the proximal sites of reactive-species generation remain elusive. A number of pathways have been identified as potential contributors, including the mitochondrial electron-transport chain, dopamine, membrane NADPH oxidases, and cytosolic flavoproteins. The most recent data have also identified nitric oxide–derived reactive nitrogen intermediates as critical contributors of protein modification and cell injury, providing potential targets for therapeutic interventions. Consideration should be also given to inappropriate regulation of iron and other divalent redox metals such as copper, as well as to redox-inactive zinc. Recent data have identified genetic defects that lead to iron accumulation in neurodegenerative diseases. Mutations in the gene that encodes the main iron-storage protein ferritin light polypeptide cause dominant adult-onset basal ganglia disease (75). Its presentation includes extrapyramidal features similar to those of Huntington disease and Parkinson disease with abnormal aggregation of ferritin and iron deposits. Abnormal iron accumulation and formation of α-synuclein inclusions that prominently feature tyrosine-nitrated α-synuclein are features of iron accumulation type 1 disease (76). This disease also presents with extrapyramidal dysfunction and a defect in the pantothenate kinase gene. As is mentioned above metals could drive the generation of strong oxidants and nitrating agents or directly be involved in the aggregation processes of proteins such as α-synuclein, amyloid, ferritin, or other proteins. Metal chelation strategies have proven effective in animal models of amyloid accumulation and are now being tested in clinical trials. On the other hand, binding of zinc to protein aggregates could potentially amplify disease by favoring the accumulation of zinc-deficient SOD in ALS.
Overall oxidative processes play a critical role in the pathogenic mechanisms of neurodegenerative diseases. Promising therapeutic interventions targeted against oxidative processes could be explored in clinical trials to eventually relieve the burden of both acute and chronic neurodegeneration.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: amyotrophic lateral sclerosis (ALS); manganese superoxide dismutase (Mn-SOD); Cu/Zn superoxide dismutase (Cu/Zn-SOD); hypochlorous acid (HOCl); nitric oxide synthase (NOS); 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP); copper chaperone for Cu/Zn superoxide dismutase (CCS).
References
- 1.Lyras L, et al. Oxidative damage to proteins, lipids, and DNA in cortical brain regions from patients with dementia with Lewy bodies. J. Neurochem. 1998;71:302–312. doi: 10.1046/j.1471-4159.1998.71010302.x. [DOI] [PubMed] [Google Scholar]
- 2.Hensley K, et al. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J. Neurosci. 1998;18:8126–8132. doi: 10.1523/JNEUROSCI.18-20-08126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratico D, et al. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann. Neurol. 2000;48:809–812. [PubMed] [Google Scholar]
- 4.Giasson BI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 5.Beal MF, et al. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 6.Bagasra O, et al. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA. 1995;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington’s disease. Brain Pathol. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moskovitz J, et al. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabbita SP, Aksenov MY, Lovell MA, Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer’s disease brain. J. Neurochem. 1999;73:1660–1666. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- 10.De Boer J, et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 11.Troy CM, Derossi D, Prochiantz A, Greene LA, Shelanski ML. Downregulation of Cu/Zn superoxide dismutase leads to cell death via the nitric oxide-peroxynitrite pathway. J. Neurosci. 1996;16:253–261. doi: 10.1523/JNEUROSCI.16-01-00253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondo T, et al. Reduction of Cu, Zn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J. Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebovitz RM, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Graham DG, Montine TJ, Ho YS. Enhanced N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in mice deficient in Cu, Zn-superoxide dismutase or glutathione peroxidase. J. Neuropathol. Exp. Neurol. 2000;59:53–61. doi: 10.1093/jnen/59.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Yokota T, et al. Delayed-onset ataxia in mice lacking alpha-tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl. Acad. Sci. USA. 2001;98:15185–15190. doi: 10.1073/pnas.261456098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sano M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 17.Desnuelle C, Dib M, Garrel C, Favier A. A double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. ALS riluzole-tocopherol Study Group. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2001;2:9–18. doi: 10.1080/146608201300079364. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, et al. Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc. Natl. Acad. Sci. USA. 2001;98:11720–11724. doi: 10.1073/pnas.171325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotiriou S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into brain and for perinatal survival. Nat. Med. 2002;8:514–517. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, et al. Cu(II) potentiation of Alzheimer Abeta neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, et al. Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease. Neuron. 2000;28:461–473. doi: 10.1016/s0896-6273(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 22.Cherny RA, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 23.Chan PH, Yang GY, Chen SF, Carlson E, Epstein CJ. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing Cu, Zn-superoxide dismutase. Ann. Neurol. 1991;29:482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- 24.Przedborski S, et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakao N, et al. Overexpressing Cu/Zn superoxide dismutase enhances survival of transplanted neurons in a rat model of Parkinson’s disease. Nat. Med. 1995;1:226–231. doi: 10.1038/nm0395-226. [DOI] [PubMed] [Google Scholar]
- 26.Keller JN, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson VL, Kizushi VM, Huang PL, Snyder SH, Dawson TM. Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J. Neurosci. 1996;16:2479–2487. doi: 10.1523/JNEUROSCI.16-08-02479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pineda JA, et al. Extracellular superoxide dismutase overexpression improves behavioral outcome from closed head injury in the mouse. J. Neurotrauma. 2001;18:625–634. doi: 10.1089/089771501750291864. [DOI] [PubMed] [Google Scholar]
- 29.Pong K, Doctrow SR, Baudry M. Prevention of 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced nitration of tyrosine hydroxylase and neurotoxicity by EUK-134, a superoxide dismutase and catalase mimetic, in cultured dopaminergic neurons. Brain Res. 2000;881:182–189. doi: 10.1016/s0006-8993(00)02841-9. [DOI] [PubMed] [Google Scholar]
- 30.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 31.Melov S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 32.Parkes TL, et al. Extension of Drosophila lifespan by overexpression of human SOD1 in motor neurons. Nat. Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 33.Swerdlow RH, et al. Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann. Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 34.Davis RE, et al. Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1997;94:4526–4531. doi: 10.1073/pnas.94.9.4526. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J. Neurosci. 2000;20:RC53. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckman, J.S. 1994. Peroxynitrite versus hydroxyl radical: the role of nitric oxide in superoxide-dependent cerebral injury. In The neurobiology of NO_ and _OH. C.C. Chiueh, D.L. Gilbert, and C.A. Colton, editors. New York Academy of Sciences. New York, New York, USA. 69–75. [DOI] [PubMed]
- 37.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J. Biol. Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 38.Brennan ML, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and meyloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 39.Hantraye P, et al. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat. Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 40.Eliasson MJ, et al. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J. Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayata C, et al. Mechanisms of reduced striatal NMDA excitotoxicity in type I nitric oxide synthase knock-out mice. J. Neurosci. 1997;17:6908–6917. doi: 10.1523/JNEUROSCI.17-18-06908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przedborski S, et al. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc. Natl. Acad. Sci. USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liberatore GT, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 44.Vodovotz Y, et al. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer’s disease. J. Exp. Med. 1996;184:1425–1433. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC. The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into central nervous system in experimental allergic encephalomyelitis through maintenance of blood-central nervous system barrier integrity. J. Immunol. 2000;165:6511–6518. doi: 10.4049/jimmunol.165.11.6511. [DOI] [PubMed] [Google Scholar]
- 46.Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 1998;53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Wu DC, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu S, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 49.Xie Z, et al. Peroxynitrite mediates neurotoxicity of amyloid beta-peptide1-42- and lipopolysaccharide-activated microglia. J. Neurosci. 2002;22:3484–3492. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 51.Manning-Bog AB, et al. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J. Biol. Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 52.Lee HJ, Shin SY, Choi C, Lee YH, Lee SJ. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J. Biol. Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- 53.Paxinou E, et al. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J. Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 55.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto M, et al. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha- synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 57.Ostrerova-Golts N, et al. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J. Neurosci. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 59.Souza JM, Daikhin E, Yudkoff M, Raman CS, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch. Biochem. Biophys. 1999;371:169–178. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- 60.Tabrizi SJ, et al. Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum. Mol. Genet. 2000;9:2683–2689. doi: 10.1093/hmg/9.18.2683. [DOI] [PubMed] [Google Scholar]
- 61.Xu J, et al. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 62.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc. Natl. Acad. Sci. USA. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee FJS, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of a-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 64.Perez RG, et al. A role for a-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lotharius J, O’Malley KL. The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J. Biol. Chem. 2000;275:38581–38588. doi: 10.1074/jbc.M005385200. [DOI] [PubMed] [Google Scholar]
- 66.Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 67.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 68.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 69.Williamson TL, et al. Toxicity of ALS-linked SOD1 mutants. Science. 2000;288:399. doi: 10.1126/science.288.5465.399a. [DOI] [PubMed] [Google Scholar]
- 70.Crow JP, et al. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J. Neurochem. 1997;69:1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- 71.Estévez AG, et al. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 72.Estévez AG, et al. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J. Neurosci. 1998;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subramaniam JR, et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat. Neurosci. 2002;5:301–307. doi: 10.1038/nn823. [DOI] [PubMed] [Google Scholar]
- 74.Cassina P, et al. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J. Neurosci. Res. 2002;67:21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- 75.Curtis AR, et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat. Genet. 2001;28:350–354. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- 76.Zhou B, et al. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat. Genet. 2001;28:345–349. doi: 10.1038/ng572. [DOI] [PubMed] [Google Scholar]