Abstract
β1-adrenergic receptor (β1AR) stimulation activates the classic cAMP/protein kinase A (PKA) pathway to regulate vital cellular processes from the change of gene expression to the control of metabolism, muscle contraction, and cell apoptosis. Here we show that sustained β1AR stimulation promotes cardiac myocyte apoptosis by activation of Ca2+/calmodulin kinase II (CaMKII), independently of PKA signaling. β1AR-induced apoptosis is resistant to inhibition of PKA by a specific peptide inhibitor, PKI14-22, or an inactive cAMP analogue, Rp-8-CPT-cAMPS. In contrast, the β1AR proapoptotic effect is associated with non–PKA-dependent increases in intracellular Ca2+ and CaMKII activity. Blocking the L-type Ca2+ channel, buffering intracellular Ca2+, or inhibiting CaMKII activity fully protects cardiac myocytes against β1AR-induced apoptosis, and overexpressing a cardiac CaMKII isoform, CaMKII-δC, markedly exaggerates the β1AR apoptotic effect. These findings indicate that CaMKII constitutes a novel PKA-independent linkage of β1AR stimulation to cardiomyocyte apoptosis that has been implicated in the overall process of chronic heart failure.
Introduction
Stimulation of β-adrenergic receptor (βAR), a prototypical G protein–coupled receptor, is broadly involved in metabolic regulation, growth control, muscle contraction, cell survival, and cell death. In the heart, βAR stimulation by catecholamines serves as the most powerful regulatory mechanism to enhance myocardial performance in response to stress or exercise by activating the classic stimulatory pathway comprising the G protein Gs, adenylyl cyclase, cAMP, and protein kinase A (PKA) (1, 2). However, sustained activation of β1AR, the predominant βAR subtype expressed in the heart, also induces cardiac myocyte apoptosis (3–6). Apoptotic heart cell death has been implicated in the overall process of myocardial remodeling and the transition from cardiac hypertrophy to chronic heart failure (7–10), an illness afflicting more than five million Americans, with a 5-year mortality greater than 80% (11). However, the mechanism underlying the β1AR apoptotic effect remains poorly understood.
Previous studies suggested that β1AR-induced cardiac myocyte apoptosis was mediated by the cAMP/PKA pathway (12, 13), the only known intracellular mechanism underlying β1AR-elicited cellular responses (1, 2). However, transgenic overexpression of type V (14) or type VI (15) adenylyl cyclase in mouse hearts does not cause cell death, although it markedly augments basal PKA activity (14) and cardiac contractility (14, 15). More ironically, in cultured cardiac myocytes or in vivo, selective β2AR subtype stimulation elicits a profound cardiac protective effect (4, 5, 16), in spite of overtly enhanced cAMP formation (16–18).
The goal of the present study is to determine the mechanism of β1AR-induced apoptosis. In addition to pharmacological approaches used in WT mouse cardiac myocytes, we created genetically “pure” β1AR experimental settings using adult cardiac myocytes from β2AR KO mice (19) or by adenovirus-mediated gene transfer (20) of the mouse β1AR in myocytes from β1β2 double knockout (DKO) mice (21). These approaches enabled us to avoid the complicated interactions between the coexisting β1AR and β2AR subtypes that exert opposing effects on cardiac cell survival and cell death (4, 5, 16). In the present study, we demonstrate that β1AR-induced cardiac myocyte apoptosis is independent of cAMP and PKA signaling, but requires a novel signaling pathway involving Ca2+ and Ca2+/calmodulin-dependent protein kinase II (CaMKII).
Methods
Materials.
Isoproterenol (ISO); norepinephrine; prazosin; propranolol; cyclosporin A; FK506; ICI 118,551; H-89; pertussis toxin (PTX); nifedipine; and thapsigargin were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Rp-8-CPT-cAMPS (Rp-cAMP) was purchased from BIOLOG Life Science Institute (La Jolla, California, USA). PKI14-22 (PKI), autocamtide-2–related inhibitory peptide (AIP), KN93, and KN92 were purchased from Calbiochem-Novabiochem Corp. (La Jolla, California, USA).
Cardiac myocyte culture and adenoviral infection.
Single cardiac myocytes were enzymatically isolated from the hearts of 2- to 3-month-old WT, β2AR KO, or β1β2 DKO male mice, and then cultured. β1β2 DKO and β2AR KO cells were infected with target gene–carrying adenoviral vectors as described previously (20). Briefly, myocytes were plated at 0.5 × 104 to 1 × 104 cells per cm2 with MEM containing 1.2 mM Ca2+ and 1% penicillin-streptomycin in culture dishes precoated with 10 μg/ml mouse laminin. Adenovirus-mediated gene transfer was implemented by adding adenoviral vectors carrying either the mouse β1AR gene (Adv-β1AR), the marker gene β-gal (Adv–β-gal), or the βARK-ct gene (Adv–βARK-ct, kindly provided by R.J. Lefkowitz and W.J. Koch, Duke University, Durham, North Carolina, USA) to β1β2 DKO cells. Alternatively, hemagglutinin-tagged (HA-tagged) CaMKII-δB vector (Adv–CaMKII-δB) or HA-tagged CaMKII-δC vector (Adv–CaMKII-δC) was added to β2AR KO cells. All transfections were at an MOI of 100. At the MOI used, almost 100% of myocytes were positively infected, as evidenced by β-gal staining (20) or GFP fluorescent signal (Adv-GFP infection, our unpublished data).
After adenoviral infection for 24 hours, culture medium was added with designated reagents, including PKA inhibitors (PKI or Rp-cAMP), CaMKII inhibitors (AIP, KN93, or KN93’s inactive analogue, KN92) 1 hour prior to β1AR stimulation by ISO. In addition, calcineurin inhibitors (cyclosporin A or FK506), βAR antagonists (propranolol or ICI 118,551), or an L-type Ca2+ channel antagonist (nifedipine), or a sarcoplasmic reticulum (SR) Ca2+ pump inhibitor (thapsigargin) were added 1 hour prior to ISO treatment (1 μM) in some subsets of experiments. All apoptosis assays were performed after ISO treatment for 24 hours unless otherwise indicated. All dishes were supplemented with ascorbic acid (100 μM; Sigma-Aldrich) to prevent ISO oxidation.
TUNEL and Hoechst staining.
Nuclear fragmentation was determined in fixed cells (70% alcohol and 30% acetone) either by incubating in 10 μM Hoechst 33342 or by TUNEL staining with apoptosis detection kits (R&D Systems Inc., Minneapolis, Minnesota, USA), as previously described (5). The percentage of TUNEL- or Hoechst-positive cells was determined by counting 500–800 cardiac myocytes in 20 randomly chosen fields in each culture dish (with cells pooled from two to three hearts). (In Figure 1, c and d, Figure 2, Figure 4c, Figure 5b, and Figure 7c, all n values refer to the number of independent experiments, and each data point shows the result from 5,000–6,000 cells, n = 4–8.) As shown previously (5), the percentage of TUNEL-positive cells is lower than that of Hoechst-positive cells because some apoptotic myocytes are detached and washed away during the TUNEL staining assay.
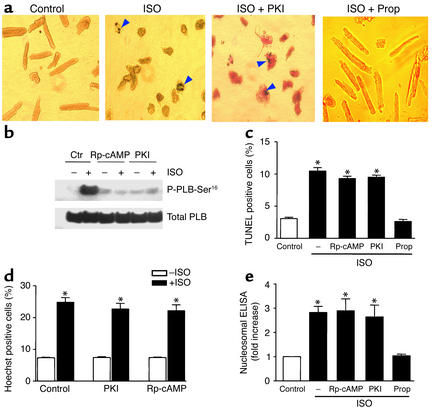
Figure 1.
Inhibition of the cAMP/PKA signaling pathway did not protect myocytes from β1AR-induced apoptosis. Cardiac myocytes from β1β2 DKO mice were infected with either Adv-β1AR or Adv–β-gal at an MOI of 100. (a) Typical micrographs of TUNEL staining of myocytes. Treatment with 1 μM ISO for 24 hours increased the number of apoptotic cells (arrows); the βAR antagonist propranolol (10 μM), but not the PKA inhibitor PKI (5 μM), prevented the β1AR apoptotic effect. (b) ISO-induced phosphorylation of PLB at Ser16 (P-PLB-Ser16) in the absence (Ctr) or presence of Rp-cAMP (100 μM) or PKI (5 μM). Similar results were obtained in four other experiments. Pretreatment periods of 1 hour (shown) and 6 hours (not shown) of cells with the PKA inhibitors were equally effective in blocking PKA-dependent PLB phosphorylation in response to ISO treatment (1 μM for 10 minutes). (c–e) Effects of PKA inhibitors on ISO-induced increase in TUNEL staining (c), Hoechst staining (d), or DNA fragmentation as assayed by cell death ELISA (e). Data are presented as mean ± SE (n = 4–8 independent experiments in 5,000–6,000 cells from 10–20 hearts for each group). *P < 0.01 vs. ISO-untreated cells or those pretreated with propranolol. Prop, propranolol.
Figure 2.
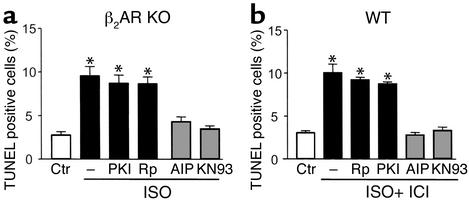
Effects of PKA and CaMKII inhibitors on β1AR-mediated increase in TUNEL-positive cells in β2AR KO (a) or WT (b) mouse cardiac myocytes. β1ARs in β2AR KO myocytes were stimulated with 1 μM ISO, and β1ARs in WT cells were stimulated with 1 μM ISO plus the β2AR blocker ICI 118,551 (0.5 μM). *P < 0.01 vs. ISO-untreated cells or those pretreated with AIP (10 μM) or KN93 (0.5 μM) (n = 6 for each group). Rp, Rp-cAMP.
Figure 4.
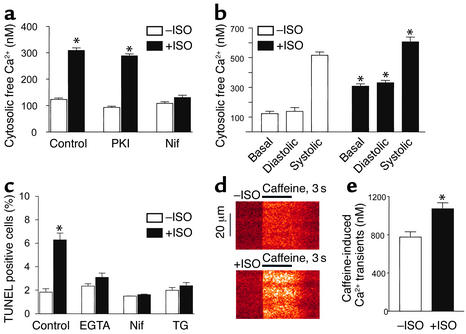
PKA-independent increase in intracellular Ca2+ is essential for the β1AR apoptotic effect. After β1β2 DKO myocytes were infected by Adv-β1AR, cells were incubated with designated reagents for 1 hour, then ISO (1 μM) was added and cells were incubated for another 3–6 hours (a, b, d, and e) or 24 hours (c). (a) Prolonged β1AR stimulation elevated basal intracellular free Ca2+ in unpaced cardiac myocytes. This effect was blocked by the L-type Ca2+ channel antagonist nifedipine (1 μM), but not the PKA inhibitor PKI (5 μM). *P < 0.01 vs. ISO-untreated groups and those pretreated by nifedipine (n = 20–35 cells from six hearts). (b) Intracellular Ca2+ transients were measured in a subset of cells electrically paced at 0.5 Hz for at least 10 minutes in the absence (n = 29 cells from four hearts) and presence (n = 22 cells from four hearts) of sustained β1AR stimulation by ISO. *P < 0.05 vs. ISO-untreated myocytes. (c) Effects of nifedipine, EGTA-AM (1 μM), or the SR ATPase inhibitor thapsigargin (1 μM) on β1AR-induced increase in TUNEL-positive cells. *P < 0.01 vs. ISO-untreated myocytes and those pretreated with EGTA-AM, nifedipine, or thapsigargin (n = 4–8). (d) Representative confocal linescan images of caffeine-elicited SR Ca2+ release in ISO-treated (1 μM, 3 hours, bottom) and untreated cells (top). The x axis shows the time courses for caffeine treatment, and the y axis shows the spatial profiles of Ca2+ transients along a scan line inside the cell. (e) Average amplitude of caffeine-elicited Ca2+ transients in ISO-treated or untreated group. *P < 0.01 vs. ISO-untreated myocytes. n = 25–30 cells from six hearts in each group. Nif, nifedipine; TG, thapsigargin.
Figure 5.
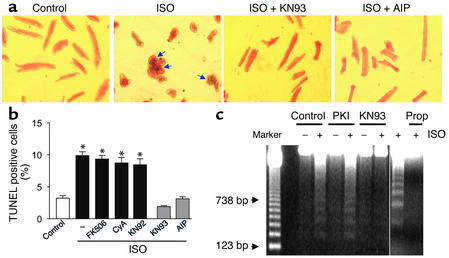
Role of CaMKII and calcineurin in the β1AR-mediated apoptotic effect. After β1β2 DKO myocytes were infected by Adv-β1AR, cells were pretreated with the CaMKII inhibitors AIP (10 μM) or KN93 (0.5 μM) or the inactive KN93 analogue KN92 (2 μM); with the calcineurin inhibitors FK506 (10 μM) or cyclosporin A (5 μM); with the PKA inhibitor PKI (5 μM); or with the Ca2+ channel blocker nifedipine (1 μM), all for 1 hour (except 3 hours for AIP) prior to administration of 1 μM ISO. Apoptosis was assessed after incubation for another 24 hours. (a) AIP or KN93 fully protected cardiomyocytes against ISO-elicited apoptosis. Arrows indicate TUNEL-positive nuclei. (b) The ISO-induced increase in TUNEL-positive cells was prevented by AIP or KN93, but not the inactive KN93 analogue KN92 or the calcineurin inhibitors FK506 or cyclosporin A. n = 4–8. *P < 0.01 versus ISO-untreated groups or those treated with KN93 or AIP. (c) ISO-induced DNA laddering in the absence (control) or presence of KN93, PKI, or the βAR antagonist propranolol. Similar results were obtained in four other experiments. CyA, cyclosporin A.
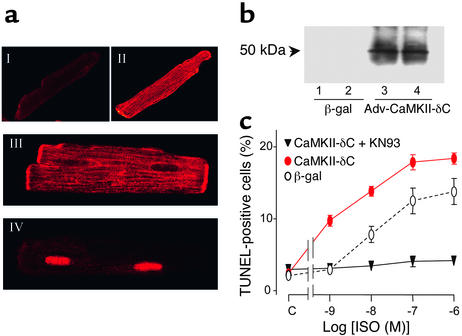
Figure 7.
Overexpression of CaMKII-δC exaggerates β1AR-induced myocyte apoptosis. (a) Confocal imaging of HA immunofluorescence in typical β2AR KO myocytes expressing either β-gal (I), or HA-tagged CaMKII-δC (II, cell surface scan; III, cell nucleus level scan), or HA-tagged CaMKII-δB (IV). (b) Expression of HA-tagged CaMKII-δC assayed by Western blot with an antibody reacting with HA. (c) Dose responses of β1AR-induced increase in apoptotic cells in β2AR KO myocytes infected by Adv–CaMKII-δC (with or without KN93) or Adv–β-gal (n = 6 for each data point).
DNA laddering and cell death ELISA.
For DNA laddering, 10 μg of DNA was loaded in each lane. The DNA was size-fractionated on a 1.5% agarose gel in Tris-acetate-EDTA buffer and then stained with ethidium bromide (Life Technologies Inc., Carlsbad, California, USA).
For cell death ELISA, myocytes in each group were harvested by trypsinization and then combined with the cells pelleted from media. The cell pellets were washed with PBS and then split into two aliquots: one used for assay of protein abundance to normalize among samples, and the other for extraction of cytoplasmic DNA. DNA was assayed using a commercially available kit to measure nucleosomal DNA (Cell Death Detection ELISA Plus; Roche Molecular Biochemicals, Indianapolis, Indiana, USA). Specifically, 0.20 ml of lysis buffer was used for each aliquot of approximately 100,000 cells. Cells were lysed by gently dispersing the pellet using a pipette tip that was cut back to prevent shearing of cells and release of nuclear DNA. The extraction was performed at room temperature and took 30 minutes. The extract was then centrifuged at low speed and assayed according to the manufacturer’s instructions. The control absorbance, measured in myocytes infected by Adv-β1AR, was considered as 100% or one unit. Fold increase was obtained by dividing the measured absorbance of an experimental group by the absorbance of the control. All measurements were normalized to the amount of total cellular protein. All measurements were made in triplicate and analysis was performed on at least four independent experiments.
Measurement of intracellular Ca2+.
Cultured myocytes in the absence of electrical pacing were loaded with a fluorescent Ca2+ probe, indo-1-acetoxymethyl ester (Molecular Probes Inc., Eugene, Oregon, USA), and were excited at 350 nm. The ratio (R) of fluorescence emission at 410 nm to that at 490 nm was used as an index of intracellular Ca2+, as described previously (22). The intracellular free Ca2+ concentration was calculated according to the equation [Ca2+]i = Kd β(R – Rmin)/(Rmax – R), where Rmin and Rmax are the ratio of fluorescence signal at 490 nm at zero and saturating [Ca2+]i, respectively; β is a constant, and Kd is the dissociation constant of the indicator. In addition, spatial properties of the caffeine-releasable SR Ca2+ store were examined using an LSM410 confocal microscope (Carl Zeiss Jena GmbH, Jena, Germany) with UV laser excitation (351 nm) and dual wavelength ratiometric (410/490 nm) imaging. In another subset of experiments, intracellular Ca2+ transients were measured in cultured myocytes electrically paced at 0.5 Hz.
Phospholamban Ser16 phosphorylation, CaMKII autophosphorylation, and CaMKII activity assay.
PKA-dependent phosphorylation of phospholamban (PLB) at Ser16 was detected by Western blot using a site-specific antibody (Badrilla, West Yorkville, United Kingdom). Total CaMKII protein abundance was assayed by Western blot using an antibody reacting with total CaMKII (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), whereas autophosphorylated CaMKII was determined with a monoclonal antibody reacting with phosphorylated CaMKII (Affinity BioReagents Inc., Golden, Colorado, USA) (23). CaMKII activity was evaluated with CaMKII assay kits (Upstate Biotechnology Inc., Lake Placid, New York, USA) and a peptide substrate (KKALRRQETVDAL) of the kinase.
Western blot analysis and confocal immunocytochemical imaging of HA-tagged CaMKII-δB or CaMKII-δC.
Adenovirus-directed expression of HA-tagged CaMKII-δB or CaMKII-δC in β2AR KO cardiomyocytes was examined by Western blot with a monoclonal antibody reacting with HA (Covance Inc., Princeton, New Jersey, USA). To determine subcellular localization of CaMKII-δC, cells were incubated overnight at 4°C with the HA monoclonal antibody, followed by an incubation for 4 hours with a Texas Red–conjugated anti-mouse antibody (Vector Laboratories Inc., Burlingame, California, USA). Immunostaining was then visualized with a scanning confocal microscope (LSM510; Carl Zeiss Jena GmbH), as described previously (23).
Statistical analysis.
Data were expressed as mean ± SE. Statistical comparisons used one-way ANOVA followed by the Bonferroni procedure for multiple-group comparisons. P < 0.05 was considered statistically significant.
Results
Sustained β1AR stimulation delivers a potent apoptotic signal in cardiac myocytes.
In β1β2 DKO cells infected with an adenoviral vector encoding β1AR (Adv-β1AR) at an MOI of 100 for 24 hours, the β1AR density was 550 ± 46 fmol/mg protein (n = 4), which was approximately 16-fold greater than the receptor density in WT cells (33.5 ± 1.5 fmol/mg protein, n = 3). Stimulation of the expressed β1ARs by ISO (1 μM) for 24 hours caused cardiomyocyte death by increased apoptosis (Figure 1a). β1AR stimulation led to a threefold augmentation in cells positive for either TUNEL (Figure 1, a and c) or Hoechst staining (Figure 1d). Apoptotic nuclei appeared blue by TUNEL staining, with a varying degree of chromatin condensation and fragmentation, as indicated by arrows (Figure 1a). Concomitantly, there was a marked increase in DNA fragmentation assayed by cell death ELISA (Figure 1e) and DNA laddering (see Figure 5c) in myocytes subjected to ISO treatment. Propranolol (10 μM), a βAR antagonist, protected myocytes from ISO-induced apoptosis (Figure 1, a, c, and e, and Figure 5c), indicating that the ISO effect is mediated by receptor activation. The earliest significant apoptotic effect occurred after 8 hours of ISO treatment, as evidenced by a 2.2-fold increase in the percentage of TUNEL-positive cells (n = 3, P < 0.05). Similarly, β1AR stimulation by ISO markedly increased apoptotic cell death in β2AR KO myocytes (Figure 2a). Furthermore, selective β1AR stimulation by ISO (1 μM) in the presence of a β2AR blocker, ICI 118,551 (0.5 μM), clearly increased WT myocyte apoptosis (Figure 2b). These results validate the relevance of our data in DKO cells expressing β1AR using adenoviral gene transfer. Thus, sustained β1AR stimulation delivers a potent apoptotic signal, in agreement with previous reports (3–6). Since a similar maximal apoptotic effect occurred in all three experimental systems examined, the receptor density appears not to be a rate-limiting factor in transducing β1AR apoptotic signal in cardiac myocytes.
β1AR-induced apoptosis is independent of cAMP/PKA signaling.
To determine the potential role of cAMP/PKA in β1AR-mediated myocyte apoptosis, we first used H-89, a widely used PKA inhibitor, and found that H-89 at a high concentration (20 μM) protected cardiac myocytes against β1AR-induced apoptosis. TUNEL-positive cells were reduced from 11.4% ± 1.1% to 3.3% ± 0.8% by H-89 (n = 4, P < 0.01), consistent with previous reports (12). However, the interpretation of experiments using H-89 is complicated by the recent finding that H-89 is also a potent βAR blocker (24). We therefore inhibited this pathway using a highly specific, membrane-permeable peptide PKA inhibitor, PKI (25), and an inactive cAMP analogue, Rp-cAMP (26). Pretreatment of myocytes with either Rp-cAMP (100 μM) or PKI (5 μM) completely abolished PKA-dependent phosphorylation of PLB (Figure 1b), a key cardiac PKA target protein that regulates the SR Ca2+ pump activity (27), thus validating the effectiveness of these PKA inhibitors in abrogating cAMP/PKA signaling. Surprisingly, neither PKA inhibitor at the same respective concentrations had any significant effect on β1AR-mediated apoptosis (Figure 1, a and c–e, and Figure 5c).
The inability of PKA inhibitors to protect cardiac myocytes against β1AR-induced apoptosis was confirmed in the β2AR KO model, in which the density and functionality of native β1ARs remain unaltered (19), and in WT mouse myocytes subjected to ISO in the presence of β2AR blockade. Stimulation of the native β1AR in adult β2AR KO or WT cardiac myocytes by ISO similarly increased TUNEL-positive cells even in the presence of PKI or Rp-cAMP (Figure 2). Thus, while the cAMP/PKA signaling pathway has been thought to be the sole mechanism responsible for β1-adrenergic responses, β1AR apoptotic signal transduction essentially bypasses this pathway.
Role of Gβγ or Gi signaling.
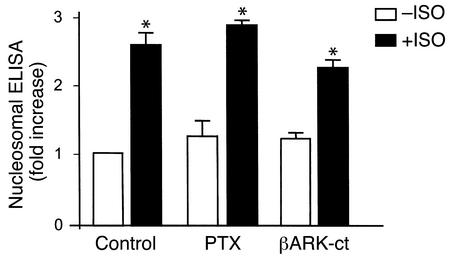
To identify the molecular mediator for the non–PKA-dependent β1AR apoptotic effect, we tested several candidates, including free Gβγ released from heterotrimeric Gs proteins or G proteins other than Gs (e.g., Gi proteins). The possible involvement of Gβγ or Gi signaling in β1AR-mediated apoptosis was examined by adenoviral gene transfer of the C-terminal domain of βAR kinase (βARK-ct) to inhibit Gβγ signaling (28) and by pretreatment of cells with PTX to disrupt Gi signaling (29). Neither βARK-ct nor PTX significantly affected β1AR-mediated apoptotic DNA fragmentation (Figure 3). In contrast, both interventions effectively blocked the Gβγ- and Gi-mediated β2AR antiapoptotic effect under similar experimental conditions (5) and prevented Gβγ-mediated activation of PI3K (30). These results rule out the possibility that Gβγ or Gi signaling is responsible for β1AR-mediated cardiomyocyte apoptosis.
Figure 3.
Gβγ or Gi signaling is not involved in β1AR-induced cardiomyocyte apoptosis. Neither inhibition of Gβγ signaling by adenoviral expression of βARK-ct nor disruption of Gi signaling by pretreatment of cells with PTX (1 μg/ml for 3 hours) altered ISO-induced (1 μM) DNA fragmentation assayed by cell death ELISA in β1β2 DKO cells infected by Adv-β1AR. *P < 0.01 vs. ISO-untreated myocytes. n = 6–7 independent experiments for each group.
Essential role of Ca2+ entry and intracellular Ca2+ in β1AR apoptotic signaling.
It has been shown that altered Ca2+ signaling promotes apoptosis in a variety of cell types (31). We next explored the possibility that Ca2+, instead of cAMP, acts as the second messenger to transmit the β1AR apoptotic signal in cardiac myocytes. In Adv-β1AR–infected DKO myocytes, ISO treatment for 3–6 hours (just prior to manifestation of β1AR-induced apoptosis) significantly elevated intracellular free Ca2+ as measured with the Ca2+-sensitive fluorescent probe indo-1. As shown in Figure 4a, sustained β1AR stimulation increased basal cytosolic Ca2+ from 122.8 ± 5.8 (n = 32 cells from six hearts) to 308.7 ± 9.7 nM (n = 29 cells from six hearts). This Ca2+ response remained largely intact in the presence of PKI (5 μM) but was abolished by nifedipine (1 μM), an L-type Ca2+ channel antagonist, indicating an elevation in intracellular Ca2+ mediated by L-type Ca2+ currents (ICa) (Figure 4a). Furthermore, we measured phasic intracellular Ca2+ transients in cultured, paced (0.5 Hz) cardiac myocytes in the presence and absence of prolonged β1AR stimulation by ISO. β1AR stimulation significantly increased both diastolic and systolic Ca2+, with the diastolic effect most prominent, in paced cardiac myocytes (Figure 4b). Thus, β1AR stimulation increases cytosolic Ca2+ in both contracting and noncontracting cardiac myocytes. In order to determine whether the altered Ca2+ homeostasis is causally linked to β1AR-induced apoptosis, we inhibited Ca2+ entry by nifedipine or buffered intracellular Ca2+ by incubating cells with EGTA-AM. Both treatments rescued cardiac myocytes from β1AR-induced apoptosis (Figure 4c).
In addition, we measured SR Ca2+ load, as indexed by the amplitude of Ca2+ transients generated in response to a bolus administration of caffeine (20 μM). We found that sustained β1AR stimulation significantly elevated the caffeine-releasable SR Ca2+ content, by 38% (Figure 4, d and e). Confocal imaging further revealed that the caffeine-induced Ca2+ release was spatially uniform (at the optical resolution), regardless of β1AR stimulation (Figure 4d). Paralyzing SR Ca2+ recycling by inhibiting the SR Ca2+ pump with thapsigargin also protected myocytes from β1AR-induced apoptosis (Figure 4c). Together, these results indicate that the enhanced L-type channel Ca2+ influx, the subsequent increases in cytosolic Ca2+, and the SR Ca2+ overload are all required for β1AR-induced myocyte apoptosis.
β1AR-induced apoptosis requires PKA-independent activation of CaMKII.
To further delineate the specific pathway transducing Ca2+-mediated β1AR apoptotic signaling, we examined possible roles of Ca2+-dependent protein phosphatases and kinases, particularly calcineurin and CaMKII. It remains controversial whether activation of calcineurin participates in Ca2+-induced cardiac myocyte apoptosis (32, 33), whereas its apoptotic effect is well established in other cell types (34). The present data show that inhibition of calcineurin by cyclosporin A (5 μM) or FK506 (10 μM) failed to block β1AR-induced apoptotic heart cell death, suggesting that calcineurin does not play an essential role in β1AR apoptotic signaling (Figure 5b). In sharp contrast, a highly specific membrane-permeable peptide inhibitor of CaMKII, autocamtide-2–related inhibitory peptide (AIP, 10 μM) (35), fully protected myocytes from β1AR-induced apoptosis, as evidenced by the typical micrographs (Figure 5a) and the average results of TUNEL staining (Figure 5b). Similar protective effects were observed with another specific CaMKII inhibitor, KN93 (0.5 μM), but not its inactive analogue, KN92 (2 μM) (Figure 5, a and b). DNA laddering assays confirmed that β1AR-induced DNA fragmentation was resistant to the PKA inhibitor PKI, but suppressed by the CaMKII inhibitor KN93 (Figure 5c). Moreover, in either β2AR KO or WT mouse cardiac myocytes, inhibition of CaMKII by AIP or KN93 similarly prevented β1AR-mediated apoptosis (Figure 2). Norepinephrine (at 1 μM), a physiological catecholamine, in the presence of an α1AR blocker, prazosin (1 μM), similarly promoted apoptosis in WT cells, an effect that was also abolished by KN93 or AIP (data not shown). These results provide the first demonstration that activation of CaMKII, rather than PKA, is required for β1AR-induced apoptosis in the heart.
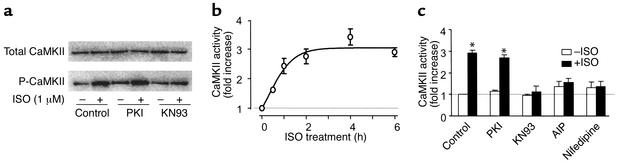
The pivotal role of CaMKII in β1AR-mediated apoptotic signaling was reinforced by data on the pharmacological profile of β1AR-induced CaMKII activation. In Adv-β1AR–infected DKO cells, ISO stimulation for 6 hours increased the level of autophosphorylated CaMKII, an active form of the kinase (36), without altering the abundance of the kinase protein (Figure 6a). Notably, the increase in CaMKII activity was PKI-resistant, but was abolished by KN93 and AIP (Figure 6, a–c) at the same respective concentrations that blocked the β1AR-mediated apoptotic effect. Since CaMKII activity was elevated prior to the manifestation of cardiomyocyte apoptosis, we conclude that CaMKII activation constitutes an early β1AR-elicited event to signal cell apoptosis.
Figure 6.
Temporal and pharmacological profiles of CaMKII activation in response to β1AR stimulation. (a) In Adv-β1AR–infected β1β2 DKO myocytes, β1AR stimulation (1 μM ISO for 6 hours) increased CaMKII autophosphorylation. This effect was blocked by KN93 (5 μM) but not by the PKA inhibitor PKI (5 μM). Similar results were obtained in three other experiments. (b) Time course of ISO-induced increase in CaMKII activity assayed by 32P incorporation into a specific peptide substrate of the kinase (see Methods; n = 4 for each data point). (c) Pharmacological profile of CaMKII activation (n = 4–6). *P < 0.01 vs. cells in the absence of ISO or those in the presence of KN93, AIP, or nifedipine. P-CaMKII, autophosphorylated CaMKII.
Overexpression of CaMKII-δC exaggerates β1AR-induced apoptosis.
Based on the aforementioned results, an increase in CaMKII abundance should enhance the β1AR apoptotic effect in cardiac myocytes. To test this hypothesis, we expressed HA-tagged CaMKII-δC, a predominant cardiac cytoplasmic CaMKII isoform (37), or CaMKII-δB, a nuclear CaMKII isoform (37), in β2AR KO myocytes. Confocal immunocytochemical analysis confirmed that the expressed CaMKII-δC was distributed in cytoplasm and the surface membranes, including the transverse tubules (Figure 7a, II and III), but not in the nuclei (Figure 7a, III), whereas CaMKII-δB was concentrated in the nuclei (Figure 7a, IV). Expression of HA-tagged CaMKII-δC was also confirmed by Western blotting using a monoclonal antibody reacting with HA (Figure 7b). Remarkably, overexpression of CaMKII-δC shifted the dose-response curve of ISO-induced apoptosis leftward by nearly an order of magnitude (EC50, 1.1 nM and 9.0 nM for myocytes infected by Adv–CaMKII-δC and Adv–β-gal, respectively) and increased the maximal apoptotic effect by 50%, whereas it exerted no appreciable apoptotic effect in the absence of β1AR stimulation by ISO (Figure 7c). Again, inhibition of CaMKII by KN93 in cardiac myocytes overexpressing CaMKII-δC abolished the β1AR apoptotic effect over a wide range of agonist concentrations (Figure 7c). By contrast, overexpression of CaMKII-δB neither enhanced nor reduced β1AR-induced myocyte apoptosis (data not shown), indicating that CaMKII-δC but not CaMKII-δB is involved in β1AR apoptotic signaling. The fact that increased CaMKII-δC abundance exaggerates the β1AR apoptotic effect without altering basal cell apoptosis substantiates the conclusion that CaMKII mediates the β1AR apoptotic signal.
Discussion
β1AR apoptotic signaling pathway.
The prevalent theory of β1AR signal transduction is that the cAMP/PKA pathway is solely responsible for β1AR-mediated cellular responses. A close inspection of studies to date, however, reveals no convincing evidence to validate that this is also the case for β1AR-evoked apoptotic signal in the heart. Using two genetically defined β1AR systems, we have provided the first documentation that sustained β1AR stimulation delivers a powerful cardiac apoptotic signal via a CaMKII-dependent, rather than a PKA-dependent, mechanism. This conclusion is based on several lines of evidence. First, inhibition of PKA by a specific peptide inhibitor, PKI, or an inactive cAMP analogue, Rp-cAMP, does not affect β1AR-induced myocyte apoptosis under conditions in which PKA-mediated protein phosphorylation is completely blocked. Second, sustained β1AR stimulation elicits PKA-independent augmentation of intracellular Ca2+ as well as SR Ca2+ load in both resting and electrically paced myocytes, and CaMKII is activated in a time-dependent fashion. More importantly, either blocking ICa, buffering intracellular Ca2+, or inhibiting CaMKII activity fully protects cardiac myocytes against β1AR-induced apoptosis. The essential role of CaMKII in β1AR apoptotic signaling is also corroborated by the fact that overexpression of a cardiac isoform of CaMKII, CaMKII-δC, markedly enhances β1AR-induced apoptosis. Similarly, in cultured adult rat cardiac myocytes, β1AR-mediated apoptosis is blocked by nifedipine or CaMKII inhibition with KN93, but not by PKA inhibition with PKI (data not shown). Thus, we conclude that the β1AR-evoked apoptotic signal is delivered by a PKA-independent, CaMKII-mediated signaling pathway. Nevertheless, this should not be taken as evidence that basal CaMKII activation (such as in the beating heart) alone is sufficient to induce apoptosis. Our previous studies have shown that 2.0-Hz electrical pacing is able to augment CaMKII activation, as shown by a twofold increase in CaMKII-dependent phosphorylation of PLB at Thr17, and that the effect of pacing on PLB-Thr17 phosphorylation is synergistically enhanced to sixfold when combined with β1AR stimulation in freshly isolated rat cardiac myocytes (38), indicating that β1AR stimulation and electrical pacing exert a synergistic effect on CaMKII activation in cardiac myocytes.
Although unexpected, the present finding that the β1AR apoptotic effect is PKA-independent is supported by previous observations that transgenic overexpression of adenylyl cyclase and the resultant elevation of intracellular cAMP in mouse hearts are dissociated from myocyte apoptosis (14, 15), and that β2AR stimulation exhibits a profound antiapoptotic effect despite elevated intracellular cAMP formation in cardiac myocytes (3–5, 16). Together, the present and previous studies indicate that an increase in cAMP/PKA signaling does not necessarily cause heart cell apoptosis. This perhaps reflects distinct compartmentation of intracellular cAMP under different circumstances (2, 26, 39–42). In contrast, the linkage of sustained β1AR stimulation to cardiac myocyte apoptosis by the multifunctional protein kinase, CaMKII, is in general agreement with recent findings that, in naive cell lines, numerous insulting factors including UV light, TNF-α (43), and protein phosphatase inhibitors (44) can activate CaMKII, which then contributes to the insult-induced apoptosis.
Distinct signaling modes underlie sustained versus acute β1AR stimulation.
Given that both Ca2+ and cAMP serve as the second messenger, β1AR signal transduction bifurcates into two pathways mediated by CaMKII and PKA, respectively. Interestingly, the two pathways are called upon in tandem to fulfill distinctly different functional roles. Acute β1AR stimulation rapidly activates the cAMP/PKA pathway, with the peak response within 1 minute (45), whereas prolonged β1AR stimulation causes desensitization of cAMP/PKA signaling within 30 minutes (1, 24). This fast cAMP/PKA response is crucial to sympathetic control over the heart rate and myocardial contraction, allowing the heart to increase its output within seconds in response to a “fight-or-flight” situation. In contrast, the newly identified CaMKII signaling is evoked gradually and appears to contribute little to acute β1AR contractile response, but is essential to the β1AR cardiac apoptotic effect.
The slow kinetics of CaMKII activation may reflect a cumulative increase in intracellular Ca2+ due to a small but persistent Ca2+ entry through L-type channels. This interpretation is consistent with the facts that CaMKII activation is blocked by the L-type channel antagonist niphedipine and that CaMKII-δC is enriched beneath cell surface membranes, thus permitting an intimate interplay between CaMKII and the L-type channel (23). Alternatively, the gradual elevation of CaMKII activity may reflect that multiple steps, e.g., upregulation of intermediate signaling components, are involved in transducing receptor signal to the kinase activation.
Regardless of the exact mechanism, the time-dependent switch of signaling modes during enduring receptor stimulation may represent a new paradigm of G protein–coupled receptor signal transduction. In this regard, it has recently been shown that β2AR stimulation induces a switch from cAMP/PKA signaling to a Gi-dependent MAPK signaling pathway (46), and that these two events are causally linked, i.e., activation of cAMP/PKA is a prerequisite for the receptor coupling to Gi proteins and the subsequent activation of the MAPK pathway (46). In the case for sustained β1AR stimulation, however, the gradual activation of CaMKII is independent of PKA signaling (Figure 6). Both examples show a time-dependent homologous regulation of G protein–coupled receptor signaling.
Regarding possible mechanisms underlying the non–PKA-dependent increase in intracellular Ca2+, it has been proposed that persistent β1AR stimulation increases ICa via a direct coupling of Gsα to the Ca2+ channel (47). More recently, it has been shown that cardiac-specific overexpression of Gsα similarly augments ICa amplitude in a PKA-independent manner (48). Both lines of evidence support the idea that PKA-independent cross-talk between Gsα and the L-type Ca2+ channel may contribute to β1AR-induced increase in intracellular Ca2+ and subsequent activation of CaMKII.
Pathophysiological relevance of β1AR apoptotic signaling.
Increasing evidence indicates that prolonged β1AR stimulation exerts a cardiotoxic effect that often outweighs the short-term gain in cardiac contractile support. Both in vivo and in vitro studies have shown that enhanced β1AR signaling by either selective receptor stimulation or receptor overexpression increases proapoptotic protein levels and promotes cardiac apoptosis (3–6). Since cardiac myocytes are terminally differentiated cells, preventing the loss of irreplaceable cells is critical for the maintenance of normal cardiac function. These studies explain, at least in part, the poor prognosis of heart failure patients with tonically elevated plasma norepinephrine, an endogenous β1AR agonist (49), as well as the beneficial effects of βAR blockers, particularly β1AR blockers in chronic heart failure (50). In light of the present findings, an increase in intracellular Ca2+ and subsequent activation of CaMKII, rather than the long-suspected cAMP/PKA cascade, are particularly cardiotoxic in terms of cardiac myocyte apoptosis. Thus, unraveling the new pathway and the novel signaling mode of β1AR signal transduction hold promise for identifying key therapeutic targets and new strategies for minimizing detrimental effects of β1AR stimulation in the failing heart.
Acknowledgments
We thank E.G. Lakatta, M. Crow, M. Mattson, A. Chelsey, N.M. Soldatov, and D.R. Abernethy for valuable discussions, and B. Ziman for excellent technical support. This work was supported by the NIH Intramural Research Program(K. Chakir, H. Cheng, and R.-P. Xiao) and by Major State Basic Research Development Program (grant no. 973) of China (R.-P. Xiao).
Footnotes
See the related Commentary beginning on page 597.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: β-adrenergic receptor (βAR); protein kinase A (PKA); Ca2+/calmodulin-dependent protein kinase II (CaMKII); isoproterenol (ISO); pertussis toxin (PTX); Rp-8-CPT-cAMPS (Rp-cAMP); protein kinase A inhibitor 14-22 (PKI); autocamtide-2–related inhibitory peptide (AIP); sarcoplasmic reticulum (SR); hemagglutinin (HA); phospholamban (PLB).
References
- 1.Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ. Model systems for the study of seven-transmembrane-segment receptors. Annu. Rev. Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 2.Xiao RP. β-adrenergic signaling in the heart: dual coupling of the β2-adrenergic receptor to Gs and Gi proteins. Sci. STKE. 2001;104:RE15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- 3.Zaugg M, et al. β-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 5.Zhu WZ, et al. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisognano JD, et al. Myocardial-directed overexpression of the human β1-adrenergic receptor in transgenic mice. J. Mol. Cell. Cardiol. 2000;32:817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 7.Kang PM, Izumo S. Apoptosis and heart failure: a critical review of the literature. Circ. Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 8.Narula J, et al. Apoptosis in myocytes in end-stage heart failure. N. Engl. J. Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 9.Olivetti G, et al. Apoptosis in the failing human heart. N. Engl. J. Med. 1997;336:1131–1341. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 10.Adams JW, et al. Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konstam MA, Remme WJ. Treatment guidelines in heart failure. Prog. Cardiovasc. Dis. 1998;41:65–72. doi: 10.1016/s0033-0620(98)80033-9. [DOI] [PubMed] [Google Scholar]
- 12.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 13.Iwai-Kanai E, et al. α- and β-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999;100:305–311. doi: 10.1161/01.cir.100.3.305. [DOI] [PubMed] [Google Scholar]
- 14.Tepe NM, et al. Altering the receptor-effector ratio by transgenic overexpression of type V adenylyl cyclase: enhanced basal catalytic activity and function without increased cardiomyocyte β-adrenergic signaling. Biochemistry. 1999;38:16706–16713. doi: 10.1021/bi991619k. [DOI] [PubMed] [Google Scholar]
- 15.Gao MH, et al. Adenylyl cyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99:1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- 16.Chesley A, et al. The β2-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through Gi-dependent coupling to phosphatidylinositol 3′-kinase. Circ. Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 17.Milano CA, et al. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 18.Zhou YY, et al. Spontaneous activation of β2- but not β1-adrenoceptors expressed in cardiac myocytes from β1β2 double knockout mice. Mol. Pharmacol. 2000;58:887–894. doi: 10.1124/mol.58.5.887. [DOI] [PubMed] [Google Scholar]
- 19.Chruscinski AJ, et al. Targeted disruption of the β2-adrenergic receptor gene. J. Biol. Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YY, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am. J. Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 21.Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J. Biol. Chem. 1999;274:16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- 22.Spurgeon HA, et al. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am. J. Physiol. 1990;258:H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- 23.Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9659–9663. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penn RB, Parent JL, Pronin AN, Panettieri RA, Jr, Benovic J. Pharmacological inhibition of protein kinases in intact cells: antagonism of β-adrenergic receptor ligand binding by H-89 reveals limitations of usefulness. J. Pharmacol. Exp. Ther. 1999;288:428–437. [PubMed] [Google Scholar]
- 25.Zheng M, et al. β2-adrenergic receptor-induced p38 MAPK activation is mediated by protein kinase A rather than by Gi or Gβγ in adult mouse cardiomyocytes. J. Biol. Chem. 2000;275:40635–40640. doi: 10.1074/jbc.M006325200. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YY, et al. Localized cAMP-dependent signaling mediates β2-adrenergic modulation of cardiac excitation-contraction coupling. Am. J. Physiol. 1997;273:H1611–H1618. doi: 10.1152/ajpheart.1997.273.3.H1611. [DOI] [PubMed] [Google Scholar]
- 27.Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ. Res. 1996;79:1059–1063. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- 28.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates Gβγ-mediated signaling. J. Biol. Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 29.Xiao RP, et al. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- 30.Jo SH, Leblais V, Wang PH, Crow MT, Xiao RP. Phosphatidylinositol 3-kinase functionally compartmentalizes the concurrent Gs signaling during β2-adrenergic stimulation. Circ. Res. 2002;91:46–53. doi: 10.1161/01.res.0000024115.67561.54. [DOI] [PubMed] [Google Scholar]
- 31.Berridge MJ, Bootman MD, Lipp P. Calcium—a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 32.Saito S, et al. β-Adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J. Biol. Chem. 2000;275:34528–34533. doi: 10.1074/jbc.M002844200. [DOI] [PubMed] [Google Scholar]
- 33.Kakita T, et al. Calcineurin pathway is required for endothelin-1-mediated protection against oxidant stress-induced apoptosis in cardiac myocytes. Circ. Res. 2001;88:1239–1246. doi: 10.1161/hh1201.091794. [DOI] [PubMed] [Google Scholar]
- 34.Wang HG, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 35.Vinogradova TM, et al. Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase II activation. Circ. Res. 2000;87:760–767. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- 36.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu. Rev. Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 37.Edman CF, Schulman H. Identification and characterization of δB-CaM kinase and δC-CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin-dependent protein kinase isoforms. . Biochim. Biophys. Acta. 1994; 1221:89–101. doi: 10.1016/0167-4889(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 38.Hagemann D, et al. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J. Biol. Chem. 2000;275:22532–22536. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- 39.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 40.Ostrom RS, et al. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J. Biol. Chem. 2001;276:42063–42069. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- 41.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 42.Kuschel M, et al. Gi protein-mediated functional compartmentalization of cardiac β2-adrenergic signaling. J. Biol. Chem. 1999;274:22048–22052. doi: 10.1074/jbc.274.31.22048. [DOI] [PubMed] [Google Scholar]
- 43.Wright SC, Schellenberger U, Ji L, Wang H, Larrick JW. Calmodulin-dependent protein kinase II mediates signal transduction in apoptosis. FASEB J. 1997;11:843–849. doi: 10.1096/fasebj.11.11.9285482. [DOI] [PubMed] [Google Scholar]
- 44.Fladmark KE, et al. Ca2+/calmodulin-dependent protein kinase II is required for microcystin-induced apoptosis. J. Biol. Chem. 2002;277:2804–2811. doi: 10.1074/jbc.M109049200. [DOI] [PubMed] [Google Scholar]
- 45.Xiao RP, et al. β2-adrenergic receptor-stimulated increase in cAMP in rat heart cells is not coupled to changes in Ca2+ dynamics, contractility, or phospholamban phosphorylation. J. Biol. Chem. 1994;269:19151–19156. [PubMed] [Google Scholar]
- 46.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 47.Yatani A, Brown AM. Rapid β-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989;245:71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]
- 48.Lader AS, et al. Cardiac Gsα overexpression enhances L-type calcium channels through an adenylyl cyclase independent pathway. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9669–9674. doi: 10.1073/pnas.95.16.9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bristow MR. β-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 50.Port JD, Bristow MR. Altered β-adrenergic receptor gene regulation and signaling in chronic heart failure. J. Mol. Cell. Cardiol. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]