Abstract
We have shown that cytotoxic T lymphocytes specific for PR1, an HLA-A2–restricted nonopeptide derived from proteinase 3, kill leukemia cells and may contribute to the elimination of chronic myelogenous leukemia (CML) after treatment with IFN or allogeneic bone marrow transplant. Some patients with persistent disease also have circulating PR1-specific T cells, however, suggesting the likelihood of immune tolerance. Here we show that both high- and low-avidity PR1-specific T cells from the peripheral blood of healthy donors can be identified and selectively expanded in vitro. Although high-avidity PR1-specific T cells killed CML more effectively than low-avidity T cells, only high-avidity T cells underwent apoptosis when stimulated with high PR1 peptide concentration or when exposed to leukemia that overexpressed proteinase 3. No high-avidity PR1-specific T cells could be identified or expanded from newly diagnosed leukemia patients, whereas low-avidity T cells were readily expanded. Circulating high-avidity PR1-specific T cells were identified in IFN-sensitive patients in cytogenetic remission, however. These results provide evidence that CML shapes the host immune response and that leukemia outgrowth may result in part from leukemia-induced selective deletion of high-avidity PR1-specific T cells.
Introduction
Immunotherapy of human cancer has shown limited success to date. This may be due to tumor escape from immune recognition by downregulation of target antigen or antigen-processing machinery (1–4), by down-modulation of recognition and stimulation molecules (5, 6), or because of the production of inhibitory cytokines (7). Antigen-specific T cell tolerance through self-tolerance pathways has also been demonstrated, mostly in animal models and rarely in humans (8–11). Our ability to study tolerance mechanisms in humans has been limited by the small number of well-defined tumor antigens and by the difficulty of detecting tumor antigen-specific T cell responses. Recently, however, an increasing number of human tumor-associated antigens have been identified (12), and the development of peptide/MHC tetramers has enabled closer study of the immune responses against those antigens (13).
We identified PR1, a nine–amino acid self-peptide derived from proteinase 3 that binds HLA-A2 as a leukemia-associated cytotoxic T lymphocyte (CTL) antigen (14). PR1-specific CTLs from healthy donors and from patients with chronic myelogenous leukemia (CML) selectively kill CML cells and acute myelogenous leukemia (AML) cells and inhibit the growth of CML progenitors proportional to proteinase 3 overexpression in the target cells (15–17). We have used PR1/HLA-A2 tetramers to identify an expanded population of PR1-specific CTLs in CML patients, and their presence correlates with a cytogenetic response to IFN treatment (18). PR1-specific CTLs (PR1-CTLs) are also present in the peripheral blood of AML patients during chemotherapy-induced remission, and have a memory phenotype (17). By purifying the PR1-CTLs, we showed that these T cells could specifically kill leukemia cells, but not healthy bone marrow cells (14, 18).
Soluble peptide/MHC tetramers can also be used to distinguish CTLs with high and low T cell receptor (TCR) affinity based on fluorescence intensity, providing a method for rapidly identifying these unique CTL populations (19, 20). CTLs with relative high- or low-affinity TCR can be elicited in vitro by coculturing the lymphocytes with low or high concentrations of target antigen, respectively (21–23). Importantly, the effector function of the resulting CTLs has been shown to correlate with TCR affinity (23). High-affinity CTLs with specificity for gag, an HIV antigen, were induced to undergo apoptosis when stimulated with high-dose peptide antigen in vitro (22), suggesting that a high viral load might lead to clonal deletion of high-affinity HIV-specific CTLs over time. Similarly, CD4+ TCR affinity has been shown to be inversely correlated to antigen dose (24), and in a murine model of CD4+ T cell autoreactivity to myelin basic protein, prevention of autoimmunity was observed after loss of high-affinity T cells and outgrowth of low-affinity T cells during exposure to high doses of antigen (25). These studies suggest there is a peripheral control mechanism preventing the expansion of high-affinity autoreactive T cells that is similar to the differential avidity model for central tolerance (26, 27). It is not known whether similar peripheral tolerance mechanisms apply to self-antigens that are also tumor antigens in humans.
In this study, we set out to determine whether we could identify PR1-specific CTLs with both high- and low-affinity TCR in the peripheral blood of healthy donors and patients with leukemia. We show that there is a selective absence of high-avidity PR1-CTLs in the peripheral blood of untreated CML patients, but that they are readily expanded along with low-affinity PR1-specific CTLs from the peripheral blood of healthy donors and that they are circulating in IFN-sensitive CML patients in cytogenetic remission. Furthermore, we show that effector function of PR1-CTLs correlate with TCR avidity and that high-avidity PR1-CTLs die by apoptosis when exposed to either high-dose peptide antigen or to CML that overexpresses proteinase 3. These results show that specific deletion of peripheral high-avidity CTLs specific for a leukemia-associated self-antigen occurs in CML patients, which may allow outgrowth of leukemia cells that overexpress the antigen.
Methods
Patients and donors.
Donors and patients were treated at the University of Texas M.D. Anderson Cancer Center on protocols approved by the Institutional Review Board. After informed consent, cells from the CML patients and their HLA-matched bone marrow (BM) transplantation donors were obtained. PBMCs from untreated CML patients or from patients receiving IFN were collected and cryopreserved for later analysis. Cells were separated using Ficoll-Hypaque gradient-density (Organon Teknika Corp., Durham, North Carolina, USA) and frozen in RPMI-1640 complete medium (CM) (25 mM HEPES buffer, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin; Life Technologies Inc., Gaithersburg, Maryland, USA) supplemented with 20% heat-inactivated pooled human AB serum (AB; Sigma-Aldrich, St. Louis, Missouri, USA) and 10% DMSO, according to standard protocols. Before use, cells were thawed, washed, and suspended in CM plus 10% AB. High-resolution HLA testing was performed by the HLA Laboratory at M.D. Anderson Cancer Center.
Peptide synthesis.
PR1 (aa 169–177) peptide (VLQELNVTV) was synthesized by Bio-Synthesis (Lewisville, Texas, USA), and the HLA-A2–restricted CMV pp65 peptide (NLVPMVATV) was synthesized by the M.D. Anderson Protein and Nucleic Acid Facility, both to a minimum of 95% purity.
Cell lines and peptide binding.
T2 cells (American Type Culture Collection, Rockville, Maryland, USA) were maintained in culture in CM plus 10% FBS (Atlanta Biologicals Inc., Norcross, Georgia, USA). PR1 peptide was incubated at increasing concentrations for 18 hours at 37°C with 100 μg/ml β2m (Sigma-Aldrich) with 106 T2 cells in 1 ml CM. Cells were washed twice with CM, stained with BB7.2 Ab, washed again, and then stained with FITC-labeled secondary Ab (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). HLA-A*0201 expression was measured by FACS.
Peptide-specific CTLs were expanded in culture using methods described previously (15, 16). Briefly, PBMCs from healthy donors or CML patients were stimulated in vitro with PR1, pp65, or flu peptides. T2 cells were washed three times in serum-free CM and incubated with peptide at the indicated peptide concentration for 2 hours in CM. The peptide-loaded T2 cells were then irradiated with 7,500 cGy, washed once, and suspended with freshly isolated PBMCs at a 1:1 to a 1:2 ratio in CM supplemented with 10% AB. After 7 days in culture, a second stimulation was performed, and the following day, 60 IU/ml of recombinant human interleukin-2 (rhIL-2) (BioSource International, Camarillo, California, USA) was added. After 14 days in culture, a third stimulation was performed, followed on day 15 by addition of rhIL-2. A fourth stimulation was performed on day 21, followed on day 22 by the addition of rhIL-2. After a total of 27–28 days in culture, the peptide-stimulated T cells were obtained and tested for peptide-specific and leukemia-specific cytotoxicity as well as for phenotypic analyses.
Tetramer synthesis.
Production of MHC/peptide tetramers was described in detail elsewhere (13, 14). Briefly, a 15–amino acid substrate peptide (BSP) for BirA-dependent biotinylation has been engineered onto the COOH terminus of HLA-A2. The A2-BSP fusion protein and human β2m were expressed in Escherichia coli and were folded in vitro with the specific peptide ligand. The properly folded MHC-peptide complexes were extensively purified using FPLC and anion exchange and biotinylated on a single lysine within the BSP using the BirA enzyme (Avidity, Denver, Colorado, USA). Tetramers were produced by mixing the biotinylated MHC-peptide complexes with phycoerythrin-conjugated (PE-conjugated) Neutravidin (Molecular Probes Inc., Eugene, Oregon, USA) at a molar ratio of 4:1. PR1 tetramers were validated by staining against a CTL line specific for PR1. CMV tetramers were validated by staining PBMCs from a CMV-immune individual.
Ab’s and flow cytometry.
For routine surface-antigen staining, 106 PBMCs were incubated at 4°C with Ab. After washing, cells were incubated with FITC-labeled CD8 (Caltag Laboratories Inc., Burlingame, California, USA) for 30 minutes on ice. Surface expression of TCR was determined with FITC-labeled TCR-αβ (PharMingen, San Diego, California, USA), and annexin V using FITC-labeled Ab (Caltag Laboratories). Proteinase 3 expression was determined with primary mouse Ab (Accurate Chemical & Scientific Corp., Westbury, New York, USA). Mouse monoclonal anti–HLA-A2.1 Ab BB7.2 and anti–HLA-ABC w6/32 were derived from culture supernatants of a hybridoma cell lines (American Tissue Culture Collection). Cells were washed and fixed in 2% paraformaldehyde and analyzed on a FACScan (Becton Dickinson Immunocytometry Systems), and data were analyzed using CELLQuest (Becton Dickinson Immunocytometry Systems) software. The minimum concentration of tetramer necessary to show distinctly different avidities was determined in titration experiments. Each tetramer reagent was titered individually and used at the optimal concentration. The tetramer concentration showing the maximal separation of fluorescence intensity of CTL populations was generally 10–20 μg/ml. Propidium iodide (PI) (Becton Dickinson Immunocytometry Systems) staining (1 μg/ml) was performed to exclude dead cells, according to the manufacturer’s instructions. A “dump” channel was used with tetramer staining to eliminate monocytes and B lymphocytes with nonspecific binding to the HLA-A2 heavy chain by staining with PerCP-labeled CD14 and CD19 (both Becton Dickinson Immunocytometry Systems).
Detailed methodology for detection of t1/2 of tetramer dissociation was described elsewhere (19). Briefly, T cells were stained for 45 minutes with PR1/HLA-A2 tetramer, washed, and cooled to 4°C. To prevent rebinding of tetramer, cells were incubated in the presence of PI (1 μg/ml) with saturating amounts of BB7.2 Ab, and flow cytometry was used to measure fluorescence decay at 10-minute intervals. A constant number of CD8+ events was acquired at each time point. Total fluorescence within the tetramer-positive gate was normalized per CD8+ cell, and the antigen-specific fluorescence was determined by subtracting the total fluorescence of control healthy donor lymphocytes from the observed total fluorescence of the lymphocyte populations following peptide stimulation. This antigen-specific fluorescence was normalized to the percentage of the total fluorescence at the initial time point and plotted on a logarithmic scale. Tetramer dissociation t1/2 was determined by plotting ln (normalized fluorescence) versus time, and is given by t1/2 = 0.693/k, where k is the slope of the normalized fluorescence decay curve determined by the method of linear least squares.
CTL cytotoxicity assay.
A semiautomated minicytotoxicity assay was used to determine specific lysis as described previously (14, 15). Briefly, effector cells were prepared in doubling dilutions from 6 × 103 to 25 × 103 cells/well and were plated in 40 μl, 60-well Terasaki trays (Robbins Scientific, Sunnyvale, California, USA) with six replicates per dilution. Target cells (T2 cells, marrow-derived leukemic cells, or BM from a healthy donor) at a concentration of 2 × 106 cells/ml were stained with 10 μg/ml of Calcein-AM (Molecular Probes Inc.) for 60 minutes at 37°C. After washing three times in CM plus 10% AB, target cells were resuspended at 105 cells/ml, and 103 target cells in 10 μl medium were added to each well containing effector cells. Wells with target cells alone and medium alone were used for maximum (max) and minimum (min.) fluorescence emission, respectively. After 4 hours’ incubation at 37°C in 5% CO2, 5 μl FluoroQuench (One Lambda Inc., Canoga Park, California, USA) was added to each well, and the trays were centrifuged for 1 minute at 60 g before measurement of fluorescence using an automated CytoFluor II plate reader (PerSeptive Biosystems, Framingham, Massachusetts, USA). The percentage of lysis was calculated as follows: % lysis = {1 – [(mean experimental mission – mean min.)/(mean max – mean min.)]} × 100%.
Results
PR1-specific CTLs with high and low TCR avidity can be elicited from healthy donors.
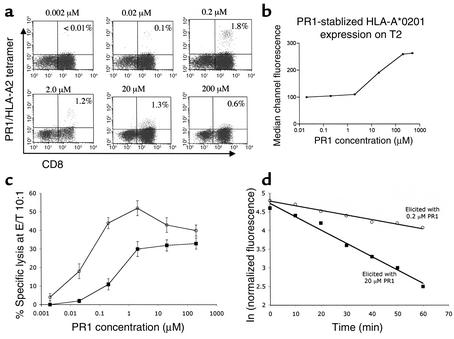
PBMCs from an HLA-A2.1+ healthy donor stimulated weekly for 28 days with 0.2 μM PR1 elicited 1.3% high-intensity PR1/HLA-A2 tetramer-staining CTLs with a median channel of fluorescence (MCF) of 293 (high-avidity PR1-CTLs), while stimulation with 20 μM PR1 produced 3.1% low-avidity (MCF = 93) PR1-specific CTLs (Figure 1a). PBMCs stimulated with 2 μM PR1elicited PR1-CTLs with intermediate TCR avidity (MCF = 211). Total TCR-αβ expression was comparable for cells elicited with 0.2, 2.0, or 20 μM PR1, suggesting that differences in tetramer staining were not due to differences in TCR expression level. Cultures stimulated with 2 μM PR1 produced fewer PR1-specific CTLs with mixed TCR avidities, while stimulation with 0.02 μM PR1 induced less than 0.1% PR1-CTLs. The efficiency of eliciting either high- or low-avidity PR1-CTLs was greatest for PBMCs stimulated with either 0.2 μM or 20 μM PR1, respectively. PR1-CTL cell numbers increased up to week 4, and similar results were obtained from PBMCs from two additional HLA-A2.1+ healthy donors (data not shown).
Figure 1.
Lower doses of PR1 peptide induce CTLs with higher intensity PR1/HLA-A2 tetramer staining that correlates with TCR avidity and inversely with effector function threshold. (a) PBMCs collected from healthy HLA-A2.1+ donors were stimulated weekly with PR1 peptide-pulsed T2 cells at the peptide concentrations indicated above each FACS plot. After 4 weeks, resulting cultures were stained with CD8 (FITC) Ab and PR1/HLA-A2 tetramer, and the percentage of CD8+ cells that stain with tetramer are noted within each FACS plot. (b) Surface HLA-A2 expression on T2 cells increases linearly with increasing concentration of PR1 peptide from 2 μM and 200 μM. T2 cells were incubated with PR1 peptide at the concentrations shown and surface HLA-A2 expression was measured by flow cytometry. (c) CTLs elicited with PR1 at 0.2 μM (open circles) or 20 μM (filled squares) PR1 were incubated for 4 hours at 37°C with PR1 peptide-pulsed T2 cells at the indicated peptide concentrations at an effector/target (E/T) ratio of 10:1 (adjusted based on the number of tetramer-positive CTLs), and percentage of specific lysis was determined. (d) Tetramer decay (t1/2) was determined to be 58 minutes and 19 minutes by plotting normalized antigen-specific fluorescence at the indicated time points for 28-day-old PR1/HLA-A2 tetramer-stained CTLs elicited with 0.2 μM (open circles) or 20 μM PR1 (filled squares), respectively. Dissociation kinetics of PR1/HLA-A2 tetramer staining were determined at 4°C in the presence of saturating concentrations of BB7.2 Ab to prevent rebinding of tetramer and in the presence of PI (1 μg/ml) to eliminate dead cells from the FACS gate.
The dose of PR1 peptide that stabilized maximal HLA-A2.1 expression on T2 cells was 200 μM, and HLA-A2.1 expression increased linearly from 2 μM to 200 μM PR1, which was approximately equivalent to the dose range of peptide that induced predominantly high- or low-avidity PR1-specific CTLs, respectively (Figure 1b). Since the same peptide-loaded T2 cell line was used to induce PR1-specific CTLs, these results indicate that a difference in the density of PR1/HLA-A2 on the APCs affects TCR avidity.
Next, T2 cells were pulsed with increasing doses of PR1 from 0.002 μM to 20 μM and combined with PR1-CTLs elicited with either low (0.2 μM) or high (20 μM) PR1 concentrations. The PR1-CTLs elicited with low PR1 showed higher specific lysis of PR1-pulsed T2 target cells than PR1-CTLs elicited with high PR1 concentration, when both were normalized at an effector–to-target ratio (E/T) of 10:1 based on the total number of PR1/HLA-A2 tetramer-positive events in the CTL cultures (Figure 1c). This shows that PR1-CTLs expanded with low concentrations of PR1 peptide have a lower threshold of activation than PR1-CTLs expanded with high PR1 concentrations, which is consistent with CTLs bearing higher and lower TCR avidities, respectively.
Since other studies have not shown a correlation of tetramer staining intensity with TCR avidity (28–30), we therefore verified our results by determining the kinetics of tetramer-staining decay using techniques described previously (19). PR1-CTLs elicited with either low (0.2 μM) or high (20 μM) PR1 concentrations for 4 weeks were incubated with PR1/HLA-A2 tetramer and saturating concentration of BB7.2 anti–HLA-A2 mAb to prevent rebinding. Normalized total fluorescence was measured at 4°C at the appropriate time points and linear tetramer-staining decay plots were obtained, indicating tetramer staining t1/2 should be proportional to the t1/2 of the respective TCR-peptide/HLA-A2 complexes (Figure 1d). PR1-CTLs elicited with low (0.2 μM) PR1 showed a threefold longer t1/2 than PR1-CTLs elicited with high (20 μM) PR1 (58 min versus 19 min), which correlates with overall high and low tetramer fluorescence (Figure 1a), respectively, and validates the use of overall tetramer fluorescence intensity to indicate relative TCR avidity.
CML target-cell killing by PR1-specific CTLs correlates with TCR avidity.
To evaluate whether CTL lines with different TCR avidities showed differences in effector function, we tested 4-week-old PR1-CTL lines derived from a healthy donor or from patients with CML for the ability to kill HLA-A2+ CML target cells from a patient with blast crisis CML, autologous chronic phase CML cells from patient CML no. 3 at time of diagnosis, or cells from healthy donors. PR1-CTLs derived from the healthy donor with high or low avidity were each combined with either BM from the patient with CML or BM from the patient’s healthy HLA-matched sibling in a 4-hour cytotoxicity assay. High-avidity PR1-CTLs elicited with 0.2 μM PR1 showed nearly twofold greater lysis of the same CML BM cells on a per-cell basis than did the low-avidity PR1-CTLs elicited with 20 μM PR1 (Figure 2a). The specific lysis of autologous BM cells by a PR1-CTL line derived from a CML patient (CML no. 3) using 0.2 μM PR1 was similar to the amount of lysis of CML BM cells by the low avidity PR1-CTL line derived from the healthy donor (Figure 2b). Similarly, CTLs from patients CML no. 1 and CML no. 2 elicited with 0.2 μM PR1 showed lysis of CML no. 3 BM cells of 24% ± 5% and 33% ± 6%, respectively, at an E/T ratio of 20:1 (not shown). There was only limited background lysis of healthy donor BM by either high- or low-avidity CTLs or by CTLs derived from the CML patients.
Figure 2.
High-avidity PR1-specific CTLs cause more specific lysis of CML BM cells than low-avidity PR1-specific CTLs. After 4 weeks in culture, PR1-stimulated CTLs were coincubated in a 4-hour microcytotoxicity assay with bone marrow cells, and specific lysis was determined. Six replicate wells were used for each dilution of effector cells. Specific lysis is plotted versus E/T ratio, and effector number was normalized for the number of PR1/HLA-A2 tetramer-staining cells in the bulk culture. (a) High-avidity PR1-specific CTLs from a healthy donor showed greater specific lysis of CML target cells than low-avidity PR1-specific CTLs. (b) PR1-specific CTL line from a CML patient 3 months after IFN treatment preferentially lyse autologous BM target cells taken at time of diagnosis over healthy HLA-A2+ BM cells from a third party, and the amount of CML target cell lysis is similar to that produced by healthy donor-derived low-avidity PR1-specific CTLs.
High-avidity PR1-specific CTLs are present only in IFN-sensitive CML patients in cytogenetic remission.
PBMCs from untreated HLA-A2+ patients with either blast crisis (CML patient no. 1) or chronic phase CML (CML no. 2) or a patient with chronic phase treated with IFN-α for 3 months (CML no. 3) were stimulated weekly with PR1. Only low-avidity PR1-CTLs could be elicited from any of the three patients (Figure 3a). There were no detectable PR1-CTLs by tetramer staining in PBMCs prior to repeated peptide stimulation. Low-avidity PR1-CTLs were elicited with as little as 0.02 μM PR1 in patient no. 2 and 0.002 μM PR1 in patient no. 3, whereas PR1-CTLs from patient no. 1 could not be elicited below 0.2 μM PR1, and overall fewer CTLs were obtained. Patients 1 and 2 had 100% Philadelphia chromosome positive (Ph+) cells in the BM by karyotype, but patient no. 3 had developed a cytogenetic response to IFN-α and had 80% Ph+ cells. Because of the possibility that high-avidity CTLs may have arisen earlier during restimulation, cultures from a fourth untreated CML patient in chronic phase (CML no. 4) were studied weekly prior to restimulation with T2 cells pulsed with 0.2 μM PR1 for the emergence of tetramer-positive CTLs, and no high-avidity PR1-CTLs emerged during culture (Figure 3b).
Figure 3.
Only low-avidity PR1-specific CTLs are elicited from peripheral blood of CML patients. PBMCs from three different HLA-A2+ CML patients were stimulated weekly with PR1-pulsed T2 cells with PR1 ranging from 0.002 μM to 200 μM. After 4 weeks, resultant cultures were stained with CD8 Ab and PR1/HLA-A2 tetramer and analyzed by FACS. The percentage of CD8+ cells that stain with relevant tetramer is indicated within each FACS plot. (a) Cultures elicited with 0.2 μM, 0.02 μM, and 0.002 μM PR1 resulted in CTLs with lower-intensity tetramer staining than CTLs from healthy donors elicited with similar doses of PR1. (b) PBMCs from an untreated chronic phase CML patient (CML no. 4) were studied weekly prior to restimulation with PR1-pulsed T2 cells with PR1/HLA-A2 tetramer. Only PR1-specific CTLs with low-intensity tetramer staining emerge over the 4 weeks, and no relatively high tetramer intensity CTLs are present. (c) PBMCs from CML no. 2 stimulated weekly with 0.2 μM pp65 peptide elicited CTLs with high-intensity pp65/HLA-A2 tetramer staining after 4 weeks in culture.
Failure to elicit high-avidity CTLs was restricted to PR1, since we could elicit high-avidity p65-specific CTLs from the CMV seropositive patient CML no. 2 using 0.2 μM pp65 peptide-pulsed T2 cells (Figure 3c).
Because we have shown previously that detection of functional PR1-CTLs in CML patients correlates with a cytogenetic response to IFN-α (14), we hypothesized that low-avidity PR1-CTLs present in high enough number may be capable of reducing the number of leukemia cells in patients that respond to IFN-α, especially after the expansions in cell numbers that we observed. To address this possibility, we studied two CML patients in a cytogenetic remission after 9 months of IFN treatment (CML no. 5 and CML no. 6 with 0% and 85% Ph+ cells, respectively), IFN-resistant patients with no cytogenetic remission (CML no. 7 and CML no. 8), and one untreated newly diagnosed patient (CML no. 9) for the presence of PR1-CTLs with high- or low-avidity TCR. High-avidity PR1-CTLs were identified in both patients in a cytogenetic remission, but in none of the IFN-resistant or untreated patients (Figure 4). However, low-avidity PR1-CTLs could be identified in the IFN-resistant patients, but totaled less than 0.1% of CD8+ cells. This suggests that low numbers of high-avidity PR1-CTLs may be sufficient to contribute to cytogenetic remission in IFN-sensitive patients, but leaves unanswered whether high numbers of low avidity PR1-CTLs may contribute to remission.
Figure 4.
High-avidity PR1-specific CTLs are identified in the peripheral blood of (a) IFN sensitive CML patients in cytogenetic remission, but not in (b) IFN-resistant or in (c) untreated newly diagnosed CML patients. PBMCs were stained with CD8, dump (CD14 + CD19), and PR1-HLA-A2 tetramer. Patients 5–8 were treated for a minimum of 9 months with IFN. The percentage of Ph+ chromosomes in a simultaneous BM specimen is indicated above each FACS plot, and the percentage of CD8+ cells with high-avidity PR1-specific CTLs is indicated within each FACS plot.
High PR1 concentration and proteinase 3–overexpressing CML cells induce apoptosis of high-avidity PR1-specific CTLs.
Because high-affinity virus-specific T cells are eliminated by target cells infected with a high viral load (21, 22), and because CML cells frequently overexpress proteinase 3 (15, 16), we hypothesized that high-avidity PR1-CTLs might be undetectable in untreated CML patients due to selective elimination by CML cells that overexpress the target antigen.
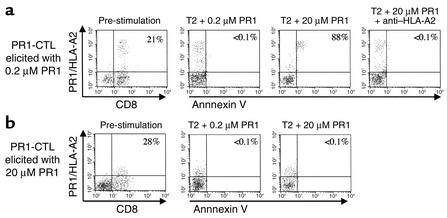
PBMCs from a second HLA-A2+ healthy donor were stimulated with either 0.2 μM or 20 μM PR1-pulsed T2 cells for 28 days to elicit high- or low-avidity PR1-CTL, respectively. Seven days after the last peptide restimulation, we challenged an equal number of CTLs with T2 cells pulsed with either high or low doses of PR1 and then studied them for evidence of apoptosis by annexin V staining 16 to 18 hours later. High-avidity PR1-CTLs underwent apoptosis when challenged with high-dose (20 μM) PR1 peptide, but not when challenged with low-dose (0.2 μM) PR1 (Figure 5). All high-avidity PR1-CTLs exposed to high-dose PR1 were dead after 36 to 48 hours of coculture. Apoptosis was abrogated in the presence of the BB7.2 blocking Ab to HLA-A2.1, and no apoptosis was observed when 20 μM of the irrelevant peptide Flu was used instead of PR1. In contrast, low-avidity PR1-CTLs did not undergo apoptosis when challenged with either high- or low-concentrations of PR1 (Figure 5b).
Figure 5.
High-avidity PR1-specific CTLs undergo apoptosis 18 hours after stimulation with high-concentration PR1 peptide. PBMCs from a healthy donor 28 days after weekly restimulation with either 0.2 μM or 20 μM PR1-pulsed T2 cells established relatively high- and low-avidity PR1-CTL, respectively (far left panels). The resulting PR1-CTLs were washed and combined in a 1:1 ratio, based on the number of tetramer-positive cells, with T2 cells pulsed with either 0.2 μM or 20 μM PR1 peptide. After 16 to 18 hours, cells were stained with annexin V Ab, and live cells were analyzed based on PI staining. The percentage of CD8+ cells that are tetramer-positive is shown in the far left panels, and the percentage of tetramer-positive cells that stain with annexin V are shown in the remaining panels. (a) Annexin V expression increased on high-avidity PR1-CTLs exposed to high-concentration (20 μM) PR1, but not after exposure to low (0.2 μM) concentration PR1. Annexin V upregulation was blocked by pretreating peptide-pulsed T2 cells with anti–HLA-A2 (BB7.2) prior to coculture with PR1-CTL. (b) Annexin V was not upregulated 18 hours after coculture of low-avidity PR1-CTLs with either low-concentration (0.2 μM) or high concentration (20 μM) PR1 peptide.
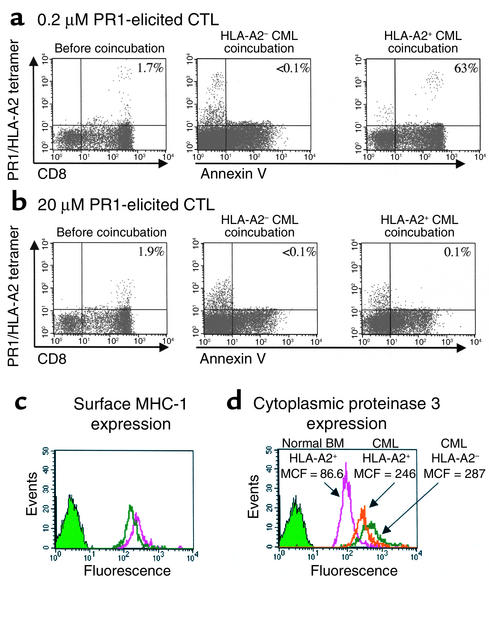
To determine whether CML cells similarly induced apoptosis of high-avidity PR1-CTL, we performed coincubation experiments with HLA-matched BM cells from CML patients, followed by staining for annexin V 16–18 hours after coincubation. High-avidity PR1-CTLs underwent apoptosis by 18 hours after coculture with BM from an HLA-matched patient with CML in chronic phase with 100% Ph+ cells (Figure 6a). No apoptosis was induced by coincubation with BM cells from an HLA-A2–negative CML patient with 100% Ph+ cells or by coincubation with BM cells from an HLA-A2+ healthy donor. In contrast, less than 1% of the low-avidity PR1-CTLs underwent apoptosis when challenged with either the HLA-A2+ or HLA-A2– CML cells (Figure 6b). MHC I expression was similar in the two patient samples (Figure 6c), and similar overexpression of cytoplasmic proteinase 3 was observed in BM cells from each of the CML patients (2.8-fold higher in the HLA-A2+ cells and 3.3-fold higher in the HLA-A2– cells compared with healthy donor BM cells; Figure 6d). Therefore, differences in apoptosis were likely due to differences in the amount of PR1 peptide presented on the CML cells.
Figure 6.
High-avidity PR1-CTLs undergo apoptosis 18 hours after coincubation with HLA-A2+ CML cells that overexpress proteinase 3. High- and low-avidity PR1-CTLs were combined in a 1:1 ratio, based upon the number of tetramer-positive cells, with CML BM cells from untreated HLA-A2+ and HLA-A2– patients. Annexin V staining was measured on live cells, based on PI staining, 18 hours after coincubation. The percentage of CD8+ cells that are tetramer-positive is shown in the left panels, and the percentage of tetramer-positive cells that stain with annexin V are shown in the remaining panels. (a) Annexin V was upregulated in the high-avidity PR1-CTLs after coincubation with HLA-A2+ cells, but not after coincubation with HLA-A2– cells. Remaining low-avidity PR1-CTLs in the culture did not upregulate annexin V. (b) In contrast, low-avidity PR1-CTLs did not upregulate annexin V after coincubation with either HLA-A2+ or HLA-A2– CML BM cells. (c) Overall MHC-I expression and proteinase 3 expression was similar in both CML BM target cells, as measured by surface staining with pan-HLA-A,B,C Ab. (d) Proteinase 3 expression was 2.8- and 3.3-fold higher in the HLA-A2+ and the HLA-A2– patient BM, respectively, compared with healthy donor BM cells.
Discussion
Our study demonstrates that distinct CTLs with relative high or low avidity for the PR1 leukemia-associated self-antigen are present in the peripheral circulation of healthy individuals and that each can be readily expanded in short-term cultures. The high-avidity PR1-CTLs are twofold more potent at killing CML cells than low-avidity PR1-CTLs. Importantly, high-avidity CTLs are selectively deleted by apoptosis after exposure to high PR1 peptide concentrations and after exposure to CML cells that overexpress proteinase 3, which could be blocked by Ab to HLA-A2. Only low-avidity CTLs are detected in the peripheral blood of patients with CML, and we were unable to elicit high-avidity PR1-CTLs from untreated CML patients despite varying culture conditions. Interestingly, high-avidity PR1-CTLs were detected with tetramer staining in unstimulated CML patients that were in a cytogenetic remission after IFN treatment. Together, these data suggest a novel escape mechanism from tumor immunity by leukemia-induced selective deletion of high-avidity effector cells that have the greatest potency against CML. Since we did not detect PR1-CTLs in unstimulated PBMCs from untreated CML patients, it is likely that proteinase 3 antigen overexpression is too low to elicit low-avidity PR1-CTLs in vivo, but it is sufficiently high to induce apoptosis of the high-avidity PR1-CTL.
Our results have important implications for tumor immunotherapy strategies. Recent vaccines use peptide or protein tumor-associated self-antigens at fixed milligram-range doses or cellular vaccines that overexpress tumor antigens to elicit immunity, but there has been limited clinical activity; none have produced significant lasting immunity (31, 32). Methods to improve immunogenicity using heteroclitic peptides or altered peptide ligands with single amino acid modifications that improve MHC binding have been more effective in eliciting T cells specific for tumor-associated self-antigens (31, 33). However, the increased peptide-MHC binding affinity might also prolong the half-life of peptide/MHC binding to TCR, which might resemble the overexpression of the native antigen. Although the altered peptide ligands might therefore eliminate high-affinity T cells, TCR affinity was not reported in these trials. The therapeutic “window” of antigen concentration presented to T cells may be critically important to stimulate optimal immunity to tumors. In addition to antigen dose, the route of administration, the delivery method (dendritic cells, peptide, protein, or DNA), and the adjuvant used are all likely to influence the eventual T cell repertoire, and future vaccine trials should be optimized for antigen doses that maximize the most potent immunity. This is likely to involve smaller amounts of antigen than is currently being used. Alternatively, adoptive cellular strategies using high-avidity T cells that have been selectively expanded ex vivo may circumvent the problem of optimizing vaccine dosing.
During the course of an immune response to repeated antigenic challenge, TCR with the highest affinity for peptide/MHC can become dominant, due to preferential expansion of cells expressing TCRs, with the slowest dissociation rates for peptide/MHC binding (19). This has been observed after secondary immunization with a fixed amount of antigen. When a malignant cell overexpressing tumor-associated self-antigens expands, however, high-affinity T cells with specificity for those tumor antigens might be selectively eliminated over time through clonal deletion. This is similar to the process of clonal exhaustion of high-affinity CD8+ T cells that occurs during viral infection (34), which has also been shown for CD4+ cells (24).
The presence of high-avidity PR1-specific lymphocytes suggests incomplete self-tolerance against proteinase 3 in healthy individuals. The autoimmune disease Wegener’s granulomatosis is associated with an antineutrophil cytoplasmic Ab (ANCA) specific for proteinase 3 (35), and T cells taken from patients with active vasculitic lesions proliferate when exposed to purified proteinase 3 (36). There are no signs of autoimmunity, however, in any of the healthy donors in this study, nor in any of the CML patients that we have shown previously to have significant numbers of circulating PR1-CTLs (14), and none were ANCA+. Although the peptides being recognized in Wegener’s granulomatosis patients are unknown, T cells from these patients do not proliferate to PR1-pulsed T2 cells (36). Since the proteinase 3 transcript is not expressed in the thymus (data not shown), peripheral tolerance mechanisms may be most important in shaping the immune response against this protein. This may occur through a gradual shaping of the T cell repertoire toward a low-avidity PR1-CTL population with a high-activation threshold, thereby preventing autoimmunity by CTLs with high-affinity TCR (25, 37).
An alternative explanation for why we are unable to elicit and detect high-avidity PR1-CTLs in untreated or IFN-resistant CML patients is that they are present below the sensitivity of tetramer staining. In titration experiments, we have determined previously the detection threshold of PR1/HLA-A2 tetramer staining to be 0.01%, which is not sensitive enough to detect very rare populations. However, high-avidity pp65-specific CTLs could be expanded from the CML patients using the same culture conditions that successfully expanded high-avidity PR1-CTLs from healthy donors, suggesting that this is not a limitation of the culture conditions. Moreover, unstimulated high-avidity PR1-CTLs were identified in IFN-sensitive patients in cytogenetic remission, which suggests that an effective immune response against CML may depend upon the expansion of high-avidity PR1-CTLs. In the unlikely scenario that high-avidity PR1-CTLs are also present in untreated and IFN-resistant CML patients, but are merely undetectable, they are not present in sufficient numbers to suppress tumor growth. Finally, cytogenetic remission after treatment with IFN correlates with an expansion of PR1-CTLs (14), but one of the three patients with a cytogenetic remission in the current study had only low-avidity CTLs. This suggests that if sufficient numbers of low avidity could be elicited, adequate antitumor activity may be possible. In support of this, a recent clinical trial using a very large number of adoptively transferred melanoma-specific T cells (1010) was associated with clinical response, although TCR affinity was not measured (38). We are currently studying more patients to address this question in leukemia patients.
Acknowledgments
This work was supported in part by grants from the Leukemia & Lymphoma Society of America (LSA6148 to J.J. Molldrem) and by the NIH (CA81247 and CA49639 to J.J. Molldrem).
Footnotes
See the related Commentary beginning on page 595.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: cytotoxic T lymphocyte (CTL); chronic myelogenous leukemia (CML); acute myelogenous leukemia (AML); T cell receptor (TCR); bone marrow (BM); complete medium (CM); AB serum (AB); recombinant human interleukin-2 (rhIL-2); phycoerythrin (PE); propidium iodide (PI); effector-to-target ratio (E/T); median channel of fluorescence (MCF); Philadelphia chromosome positive (Ph+); antineutrophil cytoplasmic Ab (ANCA).
References
- 1.Hui K, Grosveld F, Festenstein H. Rejection of transplantable AKR leukaemia cells following MHC DNA-mediated cell transformation. Nature. 1984;311:750–752. doi: 10.1038/311750a0. [DOI] [PubMed] [Google Scholar]
- 2.Kaklamanis L, et al. Loss of HLA class-I alleles, heavy chains and beta 2-microglobulin in colorectal cancer. Int. J. Cancer. 1992;51:379–385. doi: 10.1002/ijc.2910510308. [DOI] [PubMed] [Google Scholar]
- 3.Restifo NP, et al. Identification of human cancers deficient in antigen processing. J. Exp. Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeurer MJ, et al. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J. Clin. Invest. 1996;98:1633–1641. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matulonis UA, et al. Role of B7-1 in mediating an immune response to myeloid leukemia cells. Blood. 1995;85:2507–2515. [PubMed] [Google Scholar]
- 6.Munro JM. In vivo expression of the B7 costimulatory molecule by subsets of antigen-presenting cells and the malignant cells of Hodgkin’s disease. Blood. 1994;83:793–798. [PubMed] [Google Scholar]
- 7.Richter G, et al. Interleukin 10 transfected into Chinese hamster ovary cells prevents tumor growth and macrophage infiltration. Cancer Res. 1993;53:4134–4137. [PubMed] [Google Scholar]
- 8.Bogen B. Peripheral T cell tolerance as a tumor escape mechanism: deletion of CD4+ T cells specific for a monoclonal immunoglobulin idiotype secreted by a plasmacytoma. Eur. J. Immunol. 1996;26:2671–2679. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- 9.Speiser DE, et al. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J. Exp. Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staveley-O’Carroll K, et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 12.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat. Rev. Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 13.Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 14.Molldrem JJ. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat. Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 15.Molldrem J, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. [PubMed] [Google Scholar]
- 16.Molldrem JJ, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90:2529–2534. [PubMed] [Google Scholar]
- 17.Scheibenbogen C, et al. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100:2132–2137. doi: 10.1182/blood-2002-01-0163. [DOI] [PubMed] [Google Scholar]
- 18.Molldrem JJ, Lee PP, Wang C, Champlin RE, Davis MM. A PR1-human leukocyte antigen-A2 tetramer can be used to isolate low- frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chronic myelogenous leukemia. Cancer Res. 1999;59:2675–2681. [PubMed] [Google Scholar]
- 19.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 20.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 21.Alexander-Miller MA, Derby MA, Sarin A, Henkart PA, Berzofsky JA. Supraoptimal peptide-major histocompatibility complex causes a decrease in bc1-2 levels and allows tumor necrosis factor alpha receptor II- mediated apoptosis of cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1391–1399. doi: 10.1084/jem.188.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 24.Rees W, et al. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderton SM, Radu CG, Lowrey PA, Ward ES, Wraith DC. Negative selection during the peripheral immune response to antigen. J. Exp. Med. 2001;193:1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman Z, Paul WE. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10365–10369. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alam SM, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 28.Rubio-Godoy V, et al. Discrepancy between ELISPOT IFN-gamma secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10302–10307. doi: 10.1073/pnas.181348898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Echchakir H, et al. Cytotoxic T lymphocytes directed against a tumor-specific mutated antigen display similar HLA tetramer binding but distinct functional avidity and tissue distribution. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9358–9363. doi: 10.1073/pnas.142308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullock TN, Mullins DW, Colella TA, Engelhard VH. Manipulation of avidity to improve effectiveness of adoptively transferred CD8(+) T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J. Immunol. 2001;167:5824–5831. doi: 10.4049/jimmunol.167.10.5824. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nestle FO, et al. Vaccination of melanoma patients with peptide or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 33.Dyall R, et al. Heteroclitic immunization induces tumor immunity. J. Exp. Med. 1998;188:1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallimore A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams RC, et al. Epitopes on proteinase 3 recognized by antibodies from patients with Wegener’s granulomatosis. J. Immunol. 1994;152:4722–4732. [PubMed] [Google Scholar]
- 36.Ballieux BE, et al. Cell-mediated autoimmunity in patients with Wegener’s granulomatosis (WG) Clin. Exp. Immunol. 1995;100:186–193. doi: 10.1111/j.1365-2249.1995.tb03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colella TA, et al. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J. Exp. Med. 2000;191:1221–1232. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]