Abstract
Cardiac antigen–specific CD8+ T cells are involved in the autoimmune component of human myocarditis. Here, we studied the differentiation and migration of pathogenic CD8+ T cell effector cells in a new mouse model of autoimmune myocarditis. A transgenic mouse line was derived that expresses cardiac myocyte restricted membrane-bound ovalbumin (CMy-mOva). The endogenous adaptive immune system of CMy-mOva mice displays tolerance to ovalbumin. Adoptive transfer of naive CD8+ T cells from the ovalbumin-specific T cell receptor–transgenic (TCR-transgenic) OT-I strain induces myocarditis in CMy-mOva mice only after subsequent inoculation with ovalbumin-expressing vesicular stomatitis virus (VSV-Ova). OT-I effector T cells derived in vitro in the presence or absence of IL-12 were adoptively transferred into CMy-mOva mice, and the consequences were compared. Although IL-12 was not required for the generation of cytolytic and IFN-γ–producing effector T cells, only effectors primed in the presence of IL-12 infiltrated CMy-mOva hearts in significant numbers, causing lethal myocarditis. Furthermore, analysis of OT-I effectors collected from a mediastinal draining lymph node indicated that only effectors primed in vitro in the presence of IL-12 proliferated in vivo. These data demonstrate the importance of IL-12 in the differentiation of pathogenic CD8+ T cells that can cause myocarditis.
Introduction
Myocarditis and dilated cardiomyopathy are associated with enteroviruses and other viral infections. In addition to direct cytopathic effects of cardiotropic viruses, there is compelling evidence that autoimmune responses contribute to the heart disease in a significant subset of patients with myocarditis and in several animal models (1–5). Autoantibodies specific for heart antigens are prevalent in patients with myocarditis and dilated cardiomyopathy, and they are more frequently found in asymptomatic relatives of patients with cardiomyopathy than in the general population (6). Evidence for a role of self-reactive T cells in human myocarditis includes the demonstration of myocardial antigen-specific T cells in biopsies from patients with myocarditis and the induction of disease in SCID mice by transfer of blood T cells from patients with myocarditis (7). A pathogenic role for T cells in virus-induced murine myocarditis is indicated by experiments in which depletion of CD4+ or CD8+ T-cell subsets before infection with coxsackievirus B3 (CVB3) reduced the severity of disease (8). Furthermore, the myocarditic effects of CVB3 infection are partly ameliorated in CD4 knockout mice, and are further reduced in combined CD4+/CD8+ knockout mice (9). CVB3 myocarditis is also suppressed in lck knockout mice, which have decreased numbers and impaired function of mature T cells (10).
Innate immune responses that accompany viral infections are required for the development of protective adaptive immune responses and may contribute to the development of pathological autoimmune responses (11). IL-12, produced mainly by pathogen-stimulated macrophages and dendritic cells, is an important link between innate and adaptive immune responses, and it has been implicated in the pathogenesis of several autoimmune diseases (12–15) as well as myocarditis (16). Experimental autoimmune myocarditis (EAM) is induced by immunization of rodents with cardiac myosin peptides in CFA along with systemic sensitization with pertussis toxin, and it is largely mediated by CD4+ T cells (17–19). EAM appears to be dependent on IL-12, and the effects of IL-12 in this model are independent of IFN-γ (20, 21). Furthermore, in some strains of mice, the autoimmune component of virally induced myocarditis is dependent on IL-12 (4).
The clonal expansion and differentiation of naive CD8+ T cells into effector cytolytic T cells requires at least two signals provided by APCs, including peptide-MHC complexes that engage the T cell receptor and costimulatory molecules, such as CD80 or CD86, that interact with CD28 on the T cell. Previous studies have shown that IL-12 can enhance cytotoxic T lymphocyte (CTL) responses and suggest that this cytokine provides an obligatory adjuvant-like “third” signal required for the generation of CTL effectors from naive CD8+ T cells (22, 23). Since IL-12 is produced as part of the innate immune response to viral infections (11) and is reported to be important in the development of autoimmune myocarditis (13, 20, 21), we hypothesized that this cytokine may be crucial for the generation of cytopathic CD8+ effector cells that play a central role in myocarditis. We tested this hypothesis using a newly developed transgenic model of myocarditis that permits us to examine the pathogenicity of uniform populations of CD8+ T cells specific for a single antigen expressed in the myocardium.
Methods
Cardiac myocyte restricted membrane-bound ovalbumin transgenic construct.
The DNA sequence corresponding to residues 139–387 of the ovalbumin gene (GenBank accession number V00383.1) was cloned downstream to DNA encoding residues 2–118 of the human transferrin receptor (CD71, GenBank accession number XM-052730), similar to a membrane ovalbumin fusion molecule described previously (24). The CD71-ovalbumin sequence was then cloned upstream to the DNA sequence encompassing bases 942–1332 of the pcDNA3.1V5-His6-TOPO plasmid (Invitrogen, Carlsbad, California, USA), which includes polyadenylation signals. The resulting “mOva” cDNA construct was inserted into pcDNA3.1 (Invitrogen) and transfected into NIH-3T3 cells and DO.11 hybridoma cells (American Type Culture Collection, Manassas, Virginia, USA), and expression of the encoded protein was verified by immunofluorescence microscopy and flow cytometry using specific antibodies to ovalbumin and the V5 epitope (data not shown). Subsequently, this mOva construct was cloned directly downstream to a DNA segment encoding the murine cardiac α-myosin heavy-chain promoter (αMyHC-P, GenBank accession number U71441.1), kindly provided by J. Gulick (Children’s Hospital Medical Center, Cincinnati, Ohio, USA) (25). The final “αMyHC-P–mOva” DNA construct (Figure 1a) was amplified in competent bacteria, linearized with specific endonucleases, separated on agarose gel, extracted on QIAquick columns (Qiagen Inc., Valencia, California, USA), and then fully sequenced.
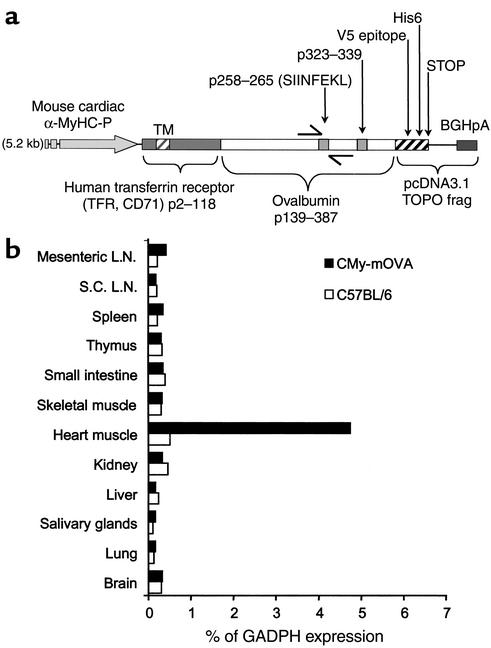
Figure 1.
CMy-mOva transgene construct and expression. (a) A map of the transgene construct used in the CMy-mOva line is shown. The Ova258–265 and Ova323–339 epitopes are recognized by OT-I and OT-II TCR transgenic T cells, respectively. BGHpA, bovine growth hormone polyadenylation signal; TM, transmembrane domain; frag, DNA fragment. (b) Real-time RT-PCR analysis of transgenic mOva RNA expression in various tissues of CMy-mOva mice. L.N., lymph nodes; S.C.L.N., subcutaneous lymph nodes.
Mice.
All mice used in the current study were bred in the pathogen-free facility at the Braunwald Medical Research Center, in accordance with the guidelines of the committee of animal research at Harvard Medical School and the NIH animal research guidelines. A transgenic mouse line we named Cardiac myocyte restricted membrane-bound ovalbumin (CMy-mOva) carrying the above-described αMyHC-P–mOva transgene was generated by a standard pronuclear microinjection directly into embryonic stem cells derived from C57BL/6 mice. All of the CMy-mOva transgenic animals used for this study originated from the same founder, were maintained on a C57BL/6-Thy 1.2 (CD90) background, and were heterozygous for the transgene. Careful examination indicated that CMy-mOva mice are healthy and have no cardiac functional abnormalities, as determined by ultrasonographic studies, or histological abnormalities for over 2 years of age. The OT-I T cell receptor (TCR) transgenic mouse strain (26) was kindly provided by W. R. Heath and F. Carbone (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) and was maintained on a C57BL/6-Thy 1.1 (CD90.1) background. The OT-I TCR is expressed on CD8+ T cells and is specific for the ovalbumin peptide p.257–264 (SIINFEKL) bound to the class I MHC molecule H2-Kb (27). Signal transducer and activator of transcription 4–/– (STAT-4–/–) mice on a C57BL/6 background (28) were provided by M. Grusby (Harvard School of Public Health, Boston, Massachusetts, USA). C57BL/6 mice used in the study were all purchased from the Jackson Laboratory (Bar Harbor, Maine, USA).
Vesicular stomatitis virus production and inoculations.
Vesicular stomatitis virus (VSV) and ovalbumin encoding VSV (VSV-Ova) were obtained from L. Lefrancois (University of Connecticut, Storrs, Connecticut, USA). Preparations and titration of VSV and VSV-Ova were performed as previously described (29). Where indicated, mice were inoculated with 106 PFU of VSV or VSV-Ova preparations through intravenous injection into the tail vein.
Cell preparations.
Spleen and lymph nodes were harvested from OT-I TCR transgenic mice, single-cell suspensions were prepared and treated with Tris-ammonium chloride (TAC) buffer to lyse erythrocytes, and CD8+ cells were purified using a MACS CD8a (Ly-2) MicroBeads kit (Miltenyi Biotec Inc., Auburn, California, USA). This protocol typically yielded over 95% CD44lowCD8+CD4– cells, as assessed by flow cytometry.
Preparation of OT-I CD8+ effector T cells was performed as described previously (30). Briefly, naive CD8+ OT-I cells were placed in culture with mitomycin-C–treated (Sigma-Aldrich, St. Louis, Missouri, USA) APCs prepared from spleens of C57BL/6 mice (31) at a T cell/APC ratio of 1:10, with 10 μM (final concentration) ovalbumin peptide antigen (SIINFEKL), with 2 μg/ml anti-CD28 (BD-Pharmingen, San Diego, California, USA); and with 50 U/ml mouse IL-2 (R&D Systems, Minneapolis, Minnesota, USA). All cultures were maintained in RPMI medium (Invitrogen) supplemented with 10% heat-inactivated FCS (Sigma), 2 mM Na-pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM HEPES (Invitrogen) in 75-cm2 flasks and were incubated at 37°C (5% CO2). In some cultures, 10 ng/ml recombinant mouse IL-12 (R&D Systems) was added at the outset, and the resulting effector populations will henceforth be referred to as OT-IIL-12 effectors. In some cultures, IL-12 was not added, but instead 2 μg/ml anti–IL-12 blocking antibody (BD-Pharmingen) was added, and the resulting effector populations will henceforth be referred to as OT-I0 effectors. All cultures were diluted 1:1 with fresh medium containing 25 U/ml IL-2 (Invitrogen) after 3 days, and cells were harvested for use at day 5. In some experiments, the OT-I cells were stimulated with splenic APCs prepared from C57BL/6 STAT-4–/– mice. In some experiments, plate-bound anti-CD3 plus soluble anti-CD28 (2 μg/ml, both reagents from BD-Pharmingen) were used to stimulate the OT-I cells in the absence of peptide or APCs.
T cell cytolytic activity assays.
Cytolytic activity of CD8+ CTL was measured against H-2Kb–expressing EL4 target cells (ATCC) pulsed with SIINFEKL by a conventional [51Cr]sodium chromate release assay (27) and by the Live/Dead Cell-Mediated Cytotoxicity Kit (Molecular Probes Inc., Eugene, Oregon, USA) according to the manufacturer’s guidelines.
Flow cytometry analyses.
Before analysis, lymphocyte preparations were washed twice in staining buffer (Dulbecco’s phosphate-buffered saline [DPBS] with 1% BSA). For phenotypic analysis of surface markers, 0.5 × 106 cells were suspended in 100 μl of staining buffer containing 1μg of each specific antibody and incubated on ice for 20 minutes followed by washing and fixation with 0.5% paraformaldehyde. Stained-cell preparations were then analyzed by flow cytometry using a FACScalibur instrument and CellQuest software (Becton Dickinson, San Jose, California, USA). Expression of the OT-I TCR was detected with anti-Vα2 and anti-Vβ5 antibodies (29). Other specific antibodies used for flow cytometry were as follows: phycoerythrin-conjugated anti-mouse CD90.1/Thy 1.1 (clone OX-7), fluorescein-conjugated anti-mouse CD44 (clone IM7), and fluorescein-conjugated anti-mouse CD25 (IL-2R α chain, clone 7D4). All antibodies were purchased from BD-Pharmingen.
In vivo proliferation of T cells was followed by flow cytometric analysis of CFSE-stained cells (Molecular Probes) as described (32). Effector cells were harvested from culture at day 5, washed, and rested in media with 10 U/ml IL-2 but no antigen for 24 hours before CFSE labeling and adoptive transfer.
ELISA detection of cytokines, cardiac troponin-T, ovalbumin protein, and specific antibodies.
To assay IFN-γ production, OT-I0 or OT-IIL-12 cells from differentiation cultures were washed twice and restimulated in 1-ml polystyrene wells coated with 5 μg of anti-CD3ε plus 2 μg/ml anti-CD28 (BD-Pharmingen), and supernatants were collected after 48 hours of culture. Assays for IL-12 were performed on supernatants of OT-I differentiation cultures sampled at 24, 48, and 72 hours. IFN-γ– or IL-12–specific ELISAs were performed using the appropriate antibody pairs (BD-Pharmingen), avidin-conjugated alkaline phosphatase (Sigma-Aldrich), and SIGMA-FAST pNPP-substrate (Sigma-Aldrich). OD measurements at 405 nm were performed on an Emax microplate reader (Molecular Devices, Sunnyvale, California, USA). Calibration curves were generated with recombinant IFN-γ or IL-12 standards (BD-Pharmingen).
For analysis of ovalbumin protein, lysates were prepared from well-perfused CMy-mOva and C57BL/6 hearts by repetitive freeze-thaw cycles followed by homogenization and centrifugation. Serial dilutions of the lysates were directly applied to ELISA plates, specific detection of ovalbumin protein was performed by incubation with purified anti-ovalbumin IgG, and subsequent detection was performed as described above, using alkaline phosphatase–conjugated antibodies.
Anti-ovalbumin IgG titers in mouse serum were determined by ELISA on microtiter plates coated with ovalbumin or an equal concentration of BSA (Sigma-Aldrich) as controls. Twofold serial dilutions of mouse sera were applied into microwells and incubated for 2 hours at 37°C, followed by washing with PBS pH 7.2 containing 0.5% Tween-20 (Sigma-Aldrich). Detection of mouse IgG specifically adsorbed to coated microwells was performed using 0.5 μg/ml alkaline phosphatase–conjugated goat anti-mouse IgG (H+L) (Southern Biotechnology Associates, Birmingham, Alabama, USA). Plates were developed as described for IFN-γ ELISA.
Serum levels of cardiac troponin-T (cTnT) were measured by a clinical quantitative sandwich enzyme immunoassay technique, which cross-reacts with mouse troponin (Troponin T STAT; Roche Diagnostics, Indianapolis, Indiana, USA) on the 1010 Elecsys Immunoanalyzer (Roche Diagnostics).
Adoptive transfer of T cells.
For adoptive transfer experiments, single-cell suspensions of naive or effector Thy 1.1+ OT-I cells in DPBS were injected into the peritoneum of recipient mice in a total volume of 0.5 ml. When indicated, cells were adoptively transferred intravenously through the tail vein in a suspension with a total volume of 100 μl.
Mouse tissue sampling and processing.
Each mouse was anesthetized by ketamine injection (0.01 ml per 10 g of body weight), the thorax was opened, the inferior vena cava was nicked, and the animals were lethally exsanguinated by perfusion of >10 ml DPBS through a 25° needle into the left ventricle. Hearts were then removed, placed in a petri dish with ice-cold RPMI medium, and cut with a scalpel to yield three transverse biventricular sections. The basal section was rapidly frozen in Tissue-Tek OCT compound (Sekura Finetek Inc., Torrance, California, USA) and stored at –80°C for subsequent immunohistochemical staining. The mid portion was fixed with 10% phosphate-buffered formalin, embedded in paraffin, and used for preparation of hematoxylin- and eosin-stained sections. The apical portion was fixed in Trizol reagent for subsequent RNA extraction. Mediastinal lymph nodes, spleen, and subcutaneous lymph nodes were removed, and single-cell suspensions were prepared for flow cytometry studies.
Grading myocarditis.
Myocarditis was graded by microscopic examination of hematoxylin- and eosin-stained sections in a blinded fashion by a trained pathologist after examination of the entire area of three sections, using a 0–4 scale as follows: 0 indicates no inflammation; 1, one to five distinct mononuclear inflammatory foci with involvement of 5% or less of the cross-sectional area; 2, more than five distinct mononuclear inflammatory foci, or involvement of over 5% but not over 20% of the cross-sectional area; 3, diffuse mononuclear inflammation involving over 20% of the area, without necrosis; and 4, diffuse inflammation with secondary necrosis and acute inflammation.
Assessment of cardiac function by ultrasonography and electrocardiography.
Transthoracic echocardiography, using a 12-MHz probe and an Agilent Sonos 4500 ultrasonograph (Philips Medical Systems, Bothell, Washington, USA), and simultaneous electrocardiography were performed as previously described (33, 34).
Immunohistochemistry.
Immunohistochemistry was performed as previously described (35). Five-micrometer-thick cryostat sections of heart were fixed in acetone, blocked with 1% BSA in PBS at room temperature, incubated with unlabeled primary antibodies (each at 1–10 μg/ml) at room temperature followed by washing in PBS, and then incubated with biotinylated secondary antibodies (each at 2.5 or 5 μg/ml) at room temperature. Primary rat anti-mouse antibodies included anti-CD4, anti-CD8, anti-Ly6-G (GR-1), anti-CD11b, and anti–class I MHC (all from BD-Pharmingen). Unlabeled isotype-matched antibodies were used as controls. The sections were then incubated with biotinylated goat anti-rat Ig (1:200; Jackson Immuno Research, West Grove, Pennsylvania, USA) at room temperature. CD90.1 (Thy 1.1) was detected using a directly biotinylated antibody (BD-Pharmingen). Sections were then blocked with 0.3% hydroperoxide/PBS at room temperature and incubated with HRP:avidin:biotin complex solutions at a 1:1:100 dilution (Vector Laboratories, Burlingame, California, USA). The antibody binding was detected with 3-amino-9-ethylcarbazole (Vector Laboratories) and counterstained with Gill’s number 2 hematoxylin solution (Polysciences Inc., Warrington, Pennsylvania, USA).
Real-time PCR.
Quantitative real-time RT-PCR was performed as described elsewhere (36). Briefly, total RNA was isolated from approximately 10 mg of heart tissue, using TRIZOL reagent (Invitrogen) T cells. To eliminate residual traces of DNA, total RNA samples were treated with DNaseI (Invitrogen). For the generation of cDNA, total RNA was quantified by absorbance at 260 nm, and equal amounts from each sample were used as templates for reverse transcription of first-strand cDNA using the ThermoScript RT-PCR system according to the manufacturer’s instructions (Invitrogen). For quantitative real-time RT-PCR analysis, 50 ng of cDNA were placed into 50-μl reaction wells in a 96-well optical reaction plate (Applied Biosystems, Foster City, California, USA) containing SYBR Green PCR mix (Applied Biosystems) and sequence-specific oligonucleotide primers (900 nM each) designed using PrimerExpres software (Applied Biosystems). The thermal cycle conditions used for all reactions were as follows: activation, 50°C for 2 minutes; denaturation, 95°C for 10 minutes; and cycle, 95°C for 15 seconds to 60°C for 1 minute (40 times). Specific primers used for sequence detection were as follows: for detection of message for the mOva transgene, 5′-AGTGGCATCAATGGCTTCT (sense) and 5′- GTTGATTATACTCTCAAGCTGCTCA (antisense); for IFN-γ, 5′-AACGCTACACACTGCATCTTGG (sense) and 5′-GCCGTGGCAGTAACAGCC (antisense); for CCR3, 5′-TGCTGTGCTACTGAGTCAGCG (sense) and 5′-CTACAGCCAGGTGGAGCAGG (antisense); for CCR5, 5′- CCATGCAGGCAACAGAGACTC (sense) and 5′-TCTCTCCAACAAAGGCATAGATGA (antisense); for CCR7, 5′- CACGCTGAGATGCTCACTGG (sense) and 5′-CCATCTGGGCCACTTGGA (antisense); and for GAPDH, 5′-GGCAAATTCAACGGCACAGT (sense) and 5′-AGATGGTGATGGGCTTCCC (antisense). All real-time reactions were carried out on an ABI 5700 Sequence Detection System (Applied Biosystems), and analysis was performed with the accompanying software. Sequences of amplicons resulting from real-time RT-PCR were verified by dissociation analysis and by sequencing. Levels of IFN-γ and mOva gene expression in tissue samples were expressed relative to endogenous levels of GAPDH expression in the same sample. All heart specimens examined in this study had nearly equal levels of GAPDH gene expression that were not affected by the treatments we applied.
Results
Tissue-specific expression of the CMy-mOva transgene.
Conventional and real-time RT-PCR analysis of RNA collected from different tissues indicates that the mOva transgene is selectively expressed only in cardiac tissue in the CMy-mOva transgenic mouse (Figure 1b). This cardiac-specific expression is consistent with other transgenic models using the same αMyHC-P (25, 37). By ELISA, we were able to detect substantial amounts of ovalbumin protein in lysates of CMy-mOva hearts but not in hearts taken from C57BL/6 control animals, and immunofluorescence staining indicated that the transgene was expressed in myocytes (data not shown).
CMy-mOva mice are tolerant to ovalbumin.
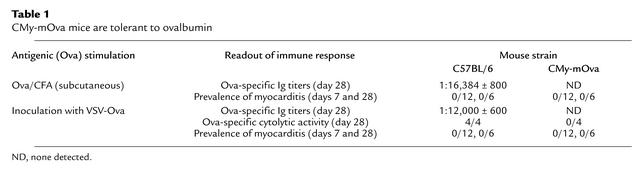
In order to determine if CMy-mOva mice are tolerant to ovalbumin as a result of expression of the transgene in the heart, we exposed the animals to exogenous ovalbumin using different routs of immunizations. CMy-mOva mice did not show humoral or cellular immune responses to ovalbumin injection in CFA or to inoculation with VSV-Ova (Table 1), whereas nontransgenic C57BL/6 mice responded strongly. Furthermore, these treatments did not lead to any inflammatory consequences in the myocardium of either CMy-mOva or control mice (Table 1).
Table 1.
CMy-mOva mice are tolerant to ovalbumin
Adoptive transfer of naive ovalbumin-specific T cells induces myocarditis in CMy-mOva mice only after VSV-Ova infection.
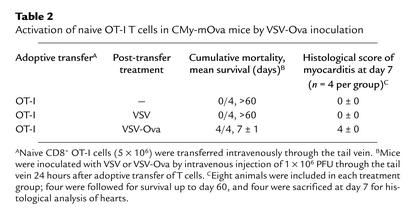
Since CMy-mOva mice are tolerant to ovalbumin expressed in the heart, the effects of adoptively transferred ovalbumin-specific T cells can be studied in isolation from the endogenous adaptive immune responses. Adoptive transfer of substantial numbers of naive CD8+ OT-I cells did not result in any detectable myocardial inflammatory process in CMy-mOva after 7, 30, or 60 days (Table 2 and data not shown). In contrast, when CMy-mOva mice were injected with naive OT-I cells and then inoculated with VSV-Ova 24 hours later, myocarditis developed and all the mice died within 8 days. Infection of CMy-mOva mice with wild-type VSV after naive OT-I transfer did not lead to myocarditis, nor did infection of C57BL/6 mice with either VSV-Ova or VSV (Table 2). These data show that exposure to a cardiac antigen in the setting of a highly immunogenic viral infection that induces a vigorous innate immune response can prime naive cardiac antigen–specific CD8+ T cells to become pathogenic effectors.
Table 2.
Activation of naive OT-I T cells in CMy-mOva mice by VSV-Ova inoculation
The influence of IL-12 on activation and differentiation of OT-I cells in vitro.
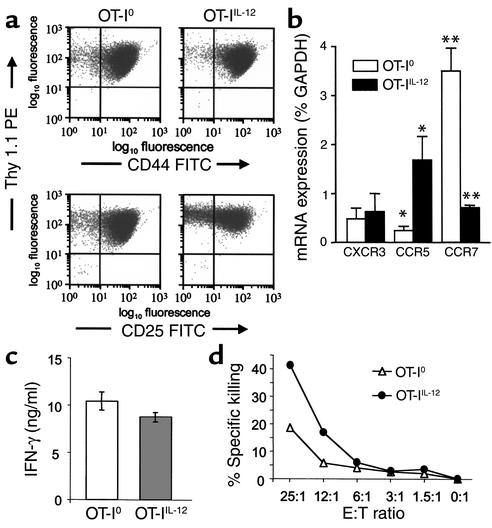
We further examined the requirements for the differentiation of pathogenic effector CTLs by activating naive OT-I cells in vitro under different conditions before adoptive transfer into CMy-mOva mice. Stimulation of naive OT-I cells in vitro with SIINFEKL, splenic APCs with or without supplementary IL-12 (10 ng/ml), resulted in an approximately 20-fold clonal expansion and differentiation into cells with an activated phenotype (CD44hiCD25+) within 5 days of culture (Figure 2a). The degree of clonal expansion and the expression of activation markers CD25 and CD44 were indistinguishable between cultures that were or were not supplemented with exogenous IL-12. By ELISA, we could not detect any endogenous IL-12 in cultures that were not supplemented with exogenous IL-12 (detection limit, <9 pg/ml) (data not shown). Moreover, there was no observed effect of 5 μg/ml anti IL-12 blocking antibody on cell yield or the phenotype of cells when added to cultures without exogenous IL-12 (data not shown). Interestingly, chemokine receptor expression, as measured by real-time RT-PCR, was different between OT-IIL-12 and OT-I0 cells. In particular, CCR5 expression was significantly higher in OT-IIL-12 cells, and CCR7 expression was significantly higher in OT-I0 cells (Figure 2b). CXCR3 expression was not significantly different between the two T-cell populations. Although IL-12 is known to promote differentiation of naive CD4+ T cells into an IFN-γ–producing “Th1” phenotype, the requirement for IL-12 in differentiation of IFN-γ–producing CD8+ effectors is not clear. For example, OT-I cells differentiated in the absence of IL-12 and the presence of IL-4 still produce IFN-γ (29). When OT-I0 and OT-IIL-12 effectors were restimulated using immobilized anti-CD3 antibody, they were equally capable of secreting IFN-γ (Figure 2c). In contrast, when the cytolytic activity of the OT-I populations were compared, we found that OT-IIL-12 effectors were approximately twice as efficient in killing SIINFEKL-loaded target cells as were OT-I0 effectors (Figure 2d).
Figure 2.
Phenotype of OT-I effector cells differentiated in vitro in the absence or presence of exogenous IL-12. Culture conditions are described in Methods. T cells were harvested from the differentiation cultures without added IL-12 (OT-I0) or with exogenously added 10 ng/ml IL-12 (OT-IIL-12) on day 5 for analysis. (a) Thy1.1, CD25, and CD44 expression by flow cytometry. PE, phycoerythrin; FITC, Fluorescein isothiocyanate. (b) Chemokine receptor expression by real-time RT-PCR; data represent means ± SEM of three separate experiments. *P = 0.037 and **P = 0.004 (t test). (c) IFN-γ secretion upon restimulation with immobilized anti-CD3 and anti-CD28. (d) Cytolytic activity against SIINFEKL-pulsed EL4 cells by a flow cytometric assay. E:T, effector:target. The data in (a), (c), and (d) are from one typical experiment out of four performed.
In vivo behavior of activated OT-I cells in CMy-mOva mice: priming in the presence of IL-12 is required for generation of pathogenic effectors.
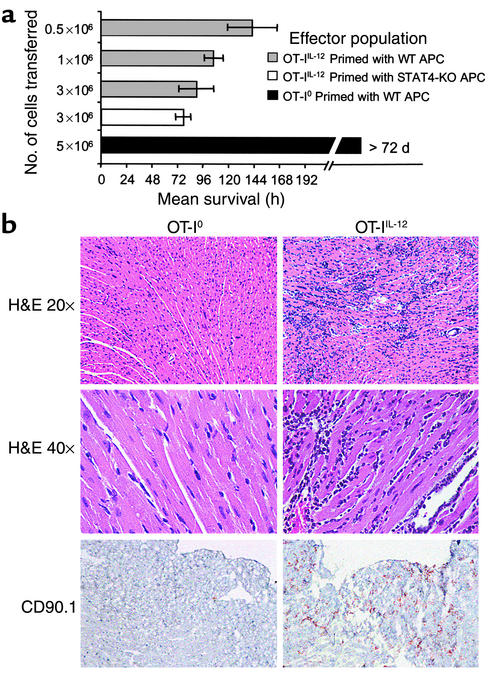
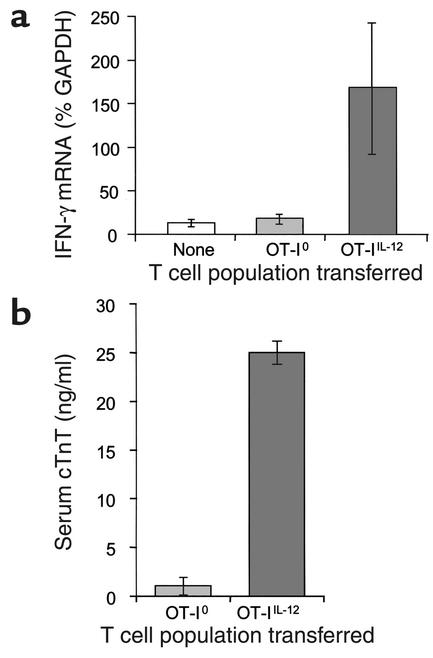
Adoptive transfer of OT-IIL-12 effectors into CMy-mOva mice consistently caused myocarditis that led to death at 96–144 hours, depending on the dose of T cells transferred (Figure 3a). The severity of myocarditis, evaluated by histological grading of hearts sampled at 72 hours after transfer, was a function of the number of T cells transferred (data not shown). Inflammation and damage to the hearts was characterized by intense lymphocytic infiltration, numerous lymphoblastic mitotic figures, myocyte apoptosis and necrosis, as well as secondary infiltration of macrophages and neutrophils (Figure 3b). No inflammation was seen in skeletal muscle or other tissues in the CMy-mOva mice with myocarditis, nor in hearts of nontransgenic C57BL/6 mice injected with the same OT-IIL-12 cells (data not shown). Immunohistochemical analysis confirmed the abundant presence of Thy1.1+ cells, which specifically mark the adoptively transferred OT-I cells in the Thy1.2 hosts (Figure 3b). By immunohistochemistry, there was a paucity of CD4+ T cells present, but foci of macrophages and granulocytes were present (data not shown). Real-time RT-PCR analysis of myocardial tissue indicated abundant IFN-γ expression in the inflamed hearts that correlated with the histological score (Figure 4a). Several chemokine mRNAs, including IP-10, MCP-1, Mig, Mip-1α, Mip-1β, and RANTES were also elevated in inflamed hearts as compared with hearts from untreated CMy-mOva mice (data not shown). Class I MHC was detected by immunohistochemistry on myocytes from CMy-mOva hearts, and there was increased expression in the setting of myocarditis (data not shown). In addition to the histology, myocyte damage could also be demonstrated by a significant increase in the serum levels of cTnT in the CMy-mOva animals that received OT-IIL-12 effectors (Figure 4b). Functional studies indicated that the mice with myocarditis had diminished contractile functioning (fractional shortening) and bradycardia with irregular rhythm. These changes correlated with the presence of T-cell infiltration and cTnT in the serum.
Figure 3.
OT-I T-cell–mediated myocarditis in CMy-mOva mice. (a) Mice were injected with indicated numbers of OT-I0 or OT-IIL-12 effector T cells, whose phenotypes are described in Figure 2, or with OT-IIL-12 cells differentiated in the presence of C57BL/6 STAT-4–/– splenic APCs. Mean survival is shown for each group. Data represent means ± SD (n = 4 per group). Additional studies with more than 16 mice confirmed that CMy-mOva mice survived longer than 72 days after adoptive transfer of 5 × 106 OT-I0. (b) Hearts were harvested 72 hours after adoptive transfer of 106 OT-I effector T cell populations (described in Figure 2). Low- and high-power photomicrographs of hematoxylin- and eosin-stained sections and immunohistochemical stains for CD90.1 (Thy 1.1), which specifically marks the OT-I cells, are shown.
Figure 4.
Cardiac IFN-γ mRNA expression and serum troponin T levels in CMy-mOva mice. (a) Total RNA was isolated from hearts removed from untreated CMy-mOva mice (labeled None) or from hearts removed 72 hours after adoptive transfer of 2.5 × 106 OT-I effectors, as indicated. Real-time RT-PCR quantification of IFN-γ mRNA was performed. Data represent means ± SD (n = 4 mice per group). (b) Troponin T was measured by ELISA in serum samples collected from CMy-mOva mice 72 hours after adoptive transfer of 2.5 × 106 OT-I effector cells. Data represent means ± SD (n = 4 mice per group).
The results of adoptive transfer of OT-I0 were strikingly different from those obtained with OT-IIL-12 cells. Transfer of OT-I0 effector cells (activated in the absence of IL-12) did not result in lethal myocarditis in CMy-mOva mice (Figure 3a). There were rare small foci of T-cell infiltration in the hearts of animals that received these cells, but there was no histological evidence of myocyte damage or secondary inflammation (Figure 3b). Furthermore, IFN-γ mRNA expression was not elevated in the hearts of these animals (Figure 4a), and these findings also correlated with the absence of cTnT in the serum (Figure 4b). Even though there was no histological evidence of significant OT-I0 cell accumulation in the hearts or myocardial damage, real-time RT-PCR analyses did indicate indirectly that at least some OT-I0 T cells had entered the heart and had some local effects. Specifically, as compared with hearts from CMy-mOva animals that did not receive T-cell transfers, hearts removed from CMy-mOva mice at72 hours after OT-I0 transfer had elevated levels of IP-10 and Mig mRNAs, similar to animals receiving OT-IIL-12 cells (data not shown). Unlike OT-IIL-12 recipients, OT-I0–recipient hearts did not have elevated levels of MCP-I, Mip-1α, Mip-1β, or RANTES mRNAs.
To determine if IL-12 was directly acting on the T cells during the differentiation of pathogenic effectors or was acting indirectly through APCs, we generated OT-IIL-12 cells using splenic APCs from C57BL/6 STAT-4–/– mice, which have impaired responses to IL-12. The effector T cells derived under these conditions were as potent in inducing lethal myocarditis as were OT-IIL-12 effectors generated in the presence of STAT-4+/+ C57BL/6 APCs (Figure 3a). We also found that IL-12 supported the differentiation of pathogenic effectors when CD8+ OT-I T cells were stimulated by the combination of immobilized anti-CD3 and soluble anti-CD28 in the absence of APCs and antigen (data not shown). Together, these findings further indicate that IL-12 acts directly on T cells rather than through effects on APCs.
In vivo proliferation and migration of OT-I effector cells.
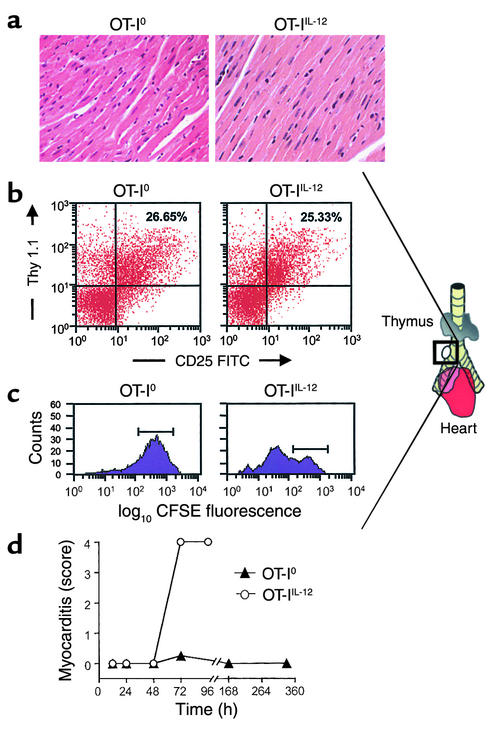
There was no detectable myocarditis in CMy-mOva hearts up to 48 hours after adoptive transfer of 3 × 106 OT-IIL-12 or OT-I0 cells (Figure 5a). Nonetheless, enlarged lymph nodes could be consistently identified in the mediastinum adjacent to the right mainstem bronchus by 48 hours after adoptive transfer of either type of OT-I effector T cell (Figure 5b). Flow cytometric analysis of cell suspensions recovered from these nodes indicated that they contained activated OT-I cells (Figure 5b). Both OT-I0 and OT-IIL-12 effectors were found with equivalent frequency and comparable CD25 expression in the mediastinal lymph nodes of CMy-mOva mice at 48 hours. The presence of OT-I cells in the lymph nodes before any evidence of myocardial inflammation in mice receiving OT-IIL-12 cells suggests that these T cells first home to the draining lymph nodes of the heart before they migrate to the myocardium. OT-I cells could not be found in extramediastinal lymph nodes (data not shown). Because both OT-I0 and OT-IIL-12 T cells found in the peribronchial lymph node at 48 hours had activated phenotypes, we wished to determine the proliferative status of these two populations. Using CFSE-labeled OT-I cells, we were able to determine that in the peribronchial lymph node, OT-IIL-12 cells had undergone significant proliferation before 72 hours, whereas OT-I0 cells had not (Figure 5c). Histological grading of myocarditis at sequential time points revealed that OT-IIL-12–mediated myocarditis developed between 48 and 72 hours after adoptive transfer. In contrast, after transfer of OT-I0 effectors, a transient infiltrate was observed in the heart at 72 hours, but there was no infiltrate seen at later time points (Figure 5d).
Figure 5.
In vivo migration and proliferation of OT-I effector cells. (a) Hematoxylin- and eosin-stained sections were prepared from hearts harvested at 48 hours after T cell transfer of 2.5 × 106 OT-I0 or OT-IIL-12 effector cells. (b) Enlarged peribronchial lymph nodes, consistently found in the illustrated location in CMy-mOva mice with myocarditis, were harvested 48 hours after adoptive transfer of 2.5 × 106 OT-I0 or OT-IIL-12 effectors, and FACS analysis was performed as described in Methods. (c) CFSE-labeled OT-I effector cells were transferred into CMy-mOva mice, and peribronchial lymph nodes were harvested after 72 hours for flow cytometric analysis. The histograms show CFSE fluorescence intensity in Thy 1.1+–gated cells. The horizontal bars indicate the range of fluorescence intensity detected in effector cells immediately after labeling, before transfer. (d) The histological grade of myocarditis was assessed, as described in Methods, in hearts harvested at various times after adoptive transfer of 3 × 106 OT-I effectors.
Discussion
This study utilizes a newly developed model of T-cell myocarditis that has allowed us to compare the in vivo behavior of different populations of T cells specific for the same defined myocardial antigen. A transgenic model of myocarditis has been described using mice expressing bacterial β-galactosidase (β-gal) under the control of a smooth-muscle cell–specific promoter (38). In those animals, myocarditis restricted to the right ventricle and accompanied by pulmonary and systemic arterial inflammation can be induced by repetitive immunization with β-gal peptide–pulsed dendritic cells. The model described here is based on transgenic models of organ-specific immunity in which OT-I TCR transgenic T cells are adoptively transferred into animals expressing transgenic ovalbumin in pancreatic islets and kidney (24, 39, 40) or intestine (41). The fact that the endogenous immune system of the CMy-mOva mice is tolerant to ovalbumin simplifies the interpretation of adoptive transfer experiments of ovalbumin-specific T cells, because secondary activation of the host adaptive immune system does not play a significant role in the pathologic processes. Adoptively transferred naive ovalbumin-specific CD8+ T cells do not spontaneously cause myocarditis in the CMy-mOva mouse, and this is consistent with the tolerant behavior of naive OT-I cells transferred to mice expressing ovalbumin in pancreatic islets or intestine (39, 41). Naive OT-I cells were activated and became pathogenic effectors in the CMy-mOva mouse after VSV-Ova inoculations, consistent with the hypothesis that innate immune responses associated with viral infection provide signals that overcome self-tolerance and lead to the generation of pathogenic effector T cells.
Recent studies using peptide immunization–induced EAM clearly indicate the importance of IL-12 in the development of autoimmune disease. Nonetheless, the influence of IL-12 on the development of pathogenic CD8+ T cells is not clarified by those studies, since EAM is predominantly a CD4+ T-cell–mediated process. The adaptive immune response to viral infections, including myocarditic viruses, includes a predominant CD8+ T cell response. Furthermore, IL-12 secretion is one component of the innate immune response to viral infections that influences the type of viral-specific adaptive immune response that will develop (42, 43). It is therefore important to know how IL-12 may influence the differentiation of pathogenic CD8+ effector T cells that can cause myocarditis. The data presented in this paper indicate that IL-12 is not required for the differentiation of IFN-γ–producing CD8+ effector T cells, but it is required for the differentiation of CD8+ effector cells that can cause cardiac damage. Although OT-I effector T cells produced abundant IFN-γ in vitro whether or not they differentiated in the presence of IL-12, there was abundant IFN-γ expression only in the hearts of CMy-mOva mice that received OT-IIL-12 cells. This probably reflects the abundance of the OT-IIL-12 cells and the scarcity of the OT-I0 cells in the hearts. Although recent reports suggest that IFN-γ is protective in EAM (21, 44), it does not appear to be protective in OT-IIL-12–mediated myocarditis in the CMy-mOva mice, since there is abundant IFN-γ expression in the hearts of animals with lethal disease.
The ability to identify and analyze draining mediastinal lymph nodes in the CMy-mOva mice represents a significant advance that, to our knowledge, has not been reported in previous studies of murine autoimmune myocarditis. The unexpected data that adoptively transferred OT-IIL-12 effector T cells home to and accumulate in draining lymph nodes before they are detectable in the heart (Figure 5) suggests that these T cells must undergo further lymph node–based activation before they are competent to infiltrate and cause damage to the heart. Furthermore, OT-IIL-12 cells proliferate in the host mice after adoptive transfer. The nonpathogenic OT-I0 cells also accumulate in the draining lymph nodes to the same extent as the OT-IIL-12 cells; however, they do not proliferate. These data indicate that OT-I0 cells are viable and activated in vivo yet fail to proliferate and migrate in significant numbers into the myocardium.
In summary, it is likely that the IL-12–induced phenotype of CD8+ T cells includes several components that render these cells more effective mediators of antimicrobial immunity and more pathogenic effectors of autoimmunity. The relatively greater pathogenicity of OT-IIL-12 cells as compared with OT-I0 cells may reflect more than one of these IL-12–induced changes. First, we find that there is a proliferative block of OT-I0 but not OT-IIL-12 cells in draining lymph nodes. Second, OT-IIL-12 cells exhibit enhanced cytolytic activity relative to OT-I0 cells, which is consistent with the results of other studies with CD8+ T cells. However, reduced lytic ability cannot explain the minimal infiltration of the cells into the heart. Third, our finding that OT-IIL-12 cells express more CCR5 and less CCR7 than OT-I0 cells may explain the relative retention of OT-I0 cells in lymph nodes and the propensity of OT-IIL-12 cells to home to heart. CCR5 binds Mip-1α, Mip-1β, and RANTES, all of which are expressed in the myocarditic hearts. CCR7 binds CCL19 and CCL21, which are present in T-cell zones of lymph nodes. The possibility of other IL-12–dependent changes in the migratory phenotype of CD8+ effectors is currently being investigated. The fact that a few OT-I0 T cells are transiently detectable in CMy-mOva hearts, and that some chemokine mRNAs are elevated in the hearts of OT-I0 recipients, suggests that the OT-I0 T cells that do get into the heart mount a weak response that cannot be sustained. Therefore, IL-12 may have additional effects, including reducing T-cell susceptibility to negative regulatory mechanisms.
Acknowledgments
This work was supported by NIH grants HL36028 (to A. H. Lichtman and N. Grabie) and HL56985 (to A. H. Lichtman). We thank Arlene Sharpe, director of the Brigham and Women’s Hospital Transgenic Core Facility, and Lina Du for pronuclear injections and derivation of the CMy-mOva transgenic founder. We thank Gary Bradwin of the Clinical and Epidemiologic Research Laboratory, Children’s Hospital, Boston, for technical assistance with the serum troponin T assays.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: cardiac myocyte restricted membrane-bound ovalbumin (CMy-mOva); T cell receptor (TCR); signal transducer and activator of transcription (STAT); vesicular stomatitis virus (VSV); coxsackievirus B3 (CVB3); experimental autoimmune myocarditis (EAM); cytotoxic T lymphocyte (CTL); α-myosin heavy-chain promoter (αMyHC-P); Tris-ammonium chloride (TAC); ovalbumin peptide antigen (SIINFEKL); Dulbecco’s phosphate-buffered saline (DPBS); cardiac troponin-T (cTnT); β-galactosidase (β-gal).
References
- 1.Huber SA. Autoimmunity in myocarditis: relevance of animal models. Clin. Immunol. Immunopathol. 1997;83:93–102. doi: 10.1006/clin.1997.4342. [DOI] [PubMed] [Google Scholar]
- 2.Huber SA. Coxsackievirus-induced myocarditis is dependent on distinct immunopathogenic responses in different strains of mice. Lab. Invest. 1997;76:691–701. [PubMed] [Google Scholar]
- 3.Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to autoimmunity. J. Autoimmun. 2001;16:175–186. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- 4.Hill SL, Rose NR. The transition from viral to autoimmune myocarditis. Autoimmunity. 2001;34:169–176. doi: 10.3109/08916930109007381. [DOI] [PubMed] [Google Scholar]
- 5.Penninger JM, Bachmaier K. Review of microbial infections and the immune response to cardiac antigens. J. Infect. Dis. 2000;181(Suppl. 3):S498–S504. doi: 10.1086/315613. [DOI] [PubMed] [Google Scholar]
- 6.Caforio AL, Goldman JH, Haven AJ, Baig KM, McKenna WJ. Evidence for autoimmunity to myosin and other heart-specific autoantigens in patients with dilated cardiomyopathy and their relatives. Int. J. Cardiol. 1996;54:157–163. doi: 10.1016/0167-5273(96)02593-4. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmbeck PL, Badorff C, Rohn G, Schulze K, Schultheiss HP. The role of sensitized T-cells in myocarditis and dilated cardiomyopathy. Int. J. Cardiol. 1996;54:117–125. doi: 10.1016/0167-5273(96)02588-0. [DOI] [PubMed] [Google Scholar]
- 8.Henke A, Huber S, Stelzner A, Whitton JL. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J. Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opavsky MA, et al. Susceptibility to myocarditis is dependent on the response of αβ T lymphocytes to coxsackieviral infection. Circ. Res. 1999;85:551–558. doi: 10.1161/01.res.85.6.551. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, et al. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat. Med. 2000;6:429–434. doi: 10.1038/74689. [DOI] [PubMed] [Google Scholar]
- 11.Xing Z, Zganiacz A, Wang J, Divangahi M, Nawaz F. Th1-type immune responses to respiratory viral infection: requirement of IL-18 for IFN-γ release in the lung but not for the differentiation of viral-reactive Th1-type lymphocytes. J. Immunol. 2000;164:2575–2584. doi: 10.4049/jimmunol.164.5.2575. [DOI] [PubMed] [Google Scholar]
- 12.Leonard J, Waldburger K, Goldman S. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J. Exp. Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarrant TK, et al. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon γ, nitric oxide, and apoptosis. J. Exp. Med. 1999;189:219–230. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neurath M, Fuss I, Kelsall B, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntyre KW, et al. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur. J. Immunol. 1996;26:2933–2938. doi: 10.1002/eji.1830261219. [DOI] [PubMed] [Google Scholar]
- 16.Okura Y, et al. Recombinant murine interleukin-12 facilitates induction of cardiac myosin-specific type 1 helper T cells in rats. Circ. Res. 1998;82:1035–1042. doi: 10.1161/01.res.82.10.1035. [DOI] [PubMed] [Google Scholar]
- 17.Pummerer C, et al. Cardiac myosin-induced myocarditis: target recognition by autoreactive T cells requires prior activation of cardiac interstitial cells. Lab. Invest. 1996;74:845–852. [PubMed] [Google Scholar]
- 18.Neumann DA, et al. In vivo deposition of myosin-specific autoantibodies in the hearts of mice with experimental autoimmune myocarditis. J. Immunol. 1992;148:3806–3813. [PubMed] [Google Scholar]
- 19.Bachmaier K, et al. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- 20.Afanasyeva M, et al. Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-γ-independent pathway. Circulation. 2001;104:3145–3151. doi: 10.1161/hc5001.100629. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/IFN-γ axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-γ. J. Immunol. 2001;167:5464–5469. doi: 10.4049/jimmunol.167.9.5464. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J. Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 23.Kieper WC, Prlic M, Schmidt CS, Mescher MF, Jameson SC. IL-12 enhances CD8 T cell homeostatic expansion. J. Immunol. 2001;166:5515–5521. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- 24.Kurts C, et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulick J, Subramaniam A, Neumann J, Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J. Biol. Chem. 1991;266:9180–9185. [PubMed] [Google Scholar]
- 26.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 27.Carbone FR, Bevan MJ. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J. Exp. Med. 1989;169:603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chitnis T, et al. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J. Clin. Invest. 2001;108:739–747. doi:10.1172/JCI200112563. doi: 10.1172/JCI12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SK, et al. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobrzanski MJ, Reome JB, Dutton RW. Type 1 and type 2 CD8+ effector T cell subpopulations promote long-term tumor immunity and protection to progressively growing tumor. J. Immunol. 2000;164:916–925. doi: 10.4049/jimmunol.164.2.916. [DOI] [PubMed] [Google Scholar]
- 31.Delfs MW, Furukawa Y, Mitchell RN, Lichtman AH. CD8+ T cell subsets TC1 and TC2 cause different histopathologic forms of murine cardiac allograft rejection. Transplantation. 2001;71:606–610. doi: 10.1097/00007890-200103150-00005. [DOI] [PubMed] [Google Scholar]
- 32.Mintern J, et al. The use of carboxyfluorescein diacetate succinimidyl ester to determine the site, duration and cell type responsible for antigen presentation in vivo. Immunol. Cell. Biol. 1999;77:539–543. doi: 10.1046/j.1440-1711.1999.00868.x. [DOI] [PubMed] [Google Scholar]
- 33.McConnell BK, et al. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J. Clin. Invest. 1999;104:1235–1244. doi: 10.1172/JCI7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fatkin D, et al. An abnormal Ca2+ response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J. Clin. Invest. 2000;106:1351–1359. doi: 10.1172/JCI11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buono C, et al. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–3031. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- 36.Wagers AJ, Waters CM, Stoolman LM, Kansas GS. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on α1,3-fucosyltransferase VII gene expression. J. Exp. Med. 1998;188:2225–2231. doi: 10.1084/jem.188.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryant D, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-α. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 38.Ludewig B, et al. Linking immune-mediated arterial inflammation and cholesterol-induced atherosclerosis in a transgenic mouse model. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12752–12757. doi: 10.1073/pnas.220427097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurts C, et al. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J. Exp. Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurts C, Kosaka H, Carbone FR, Miller JFAP, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 42.Biron CA, Gazzinelli RT. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr. Opin. Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 43.Romani L, Puccetti P, Bistoni F. Interleukin-12 in infectious diseases. Clin. Microbiol. Rev. 1997;10:611–636. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henke A, Zell R, Ehrlich G, Stelzner A. Expression of immunoregulatory cytokines by recombinant coxsackievirus B3 variants confers protection against virus-caused myocarditis. J. Virol. 2001;75:8187–8194. doi: 10.1128/JVI.75.17.8187-8194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]