Abstract
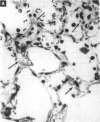
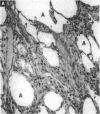
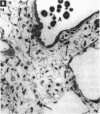
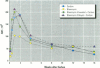
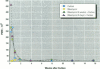
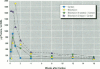
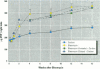
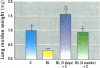
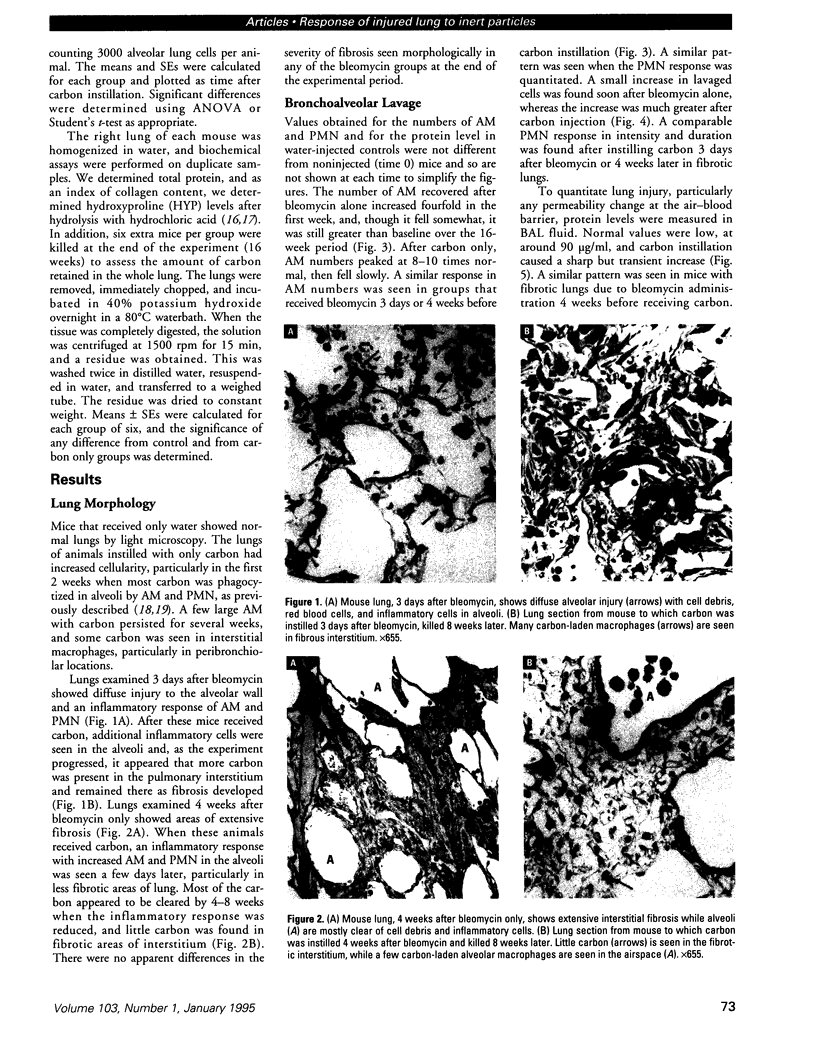
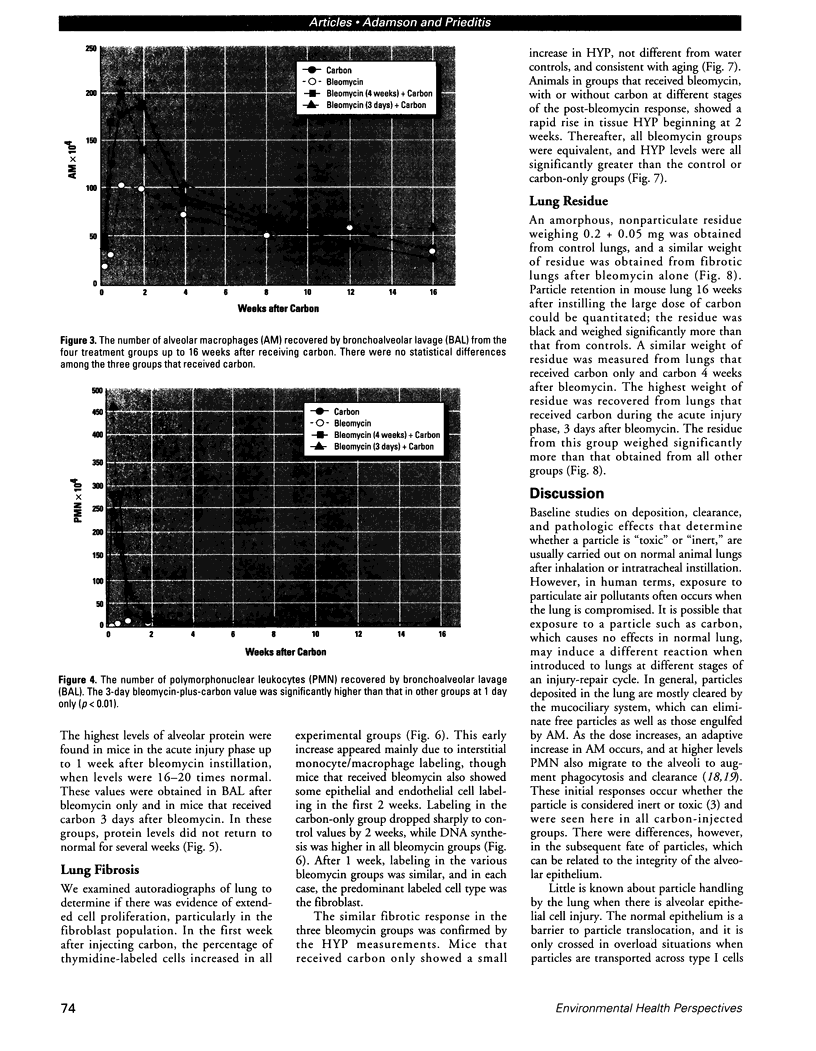
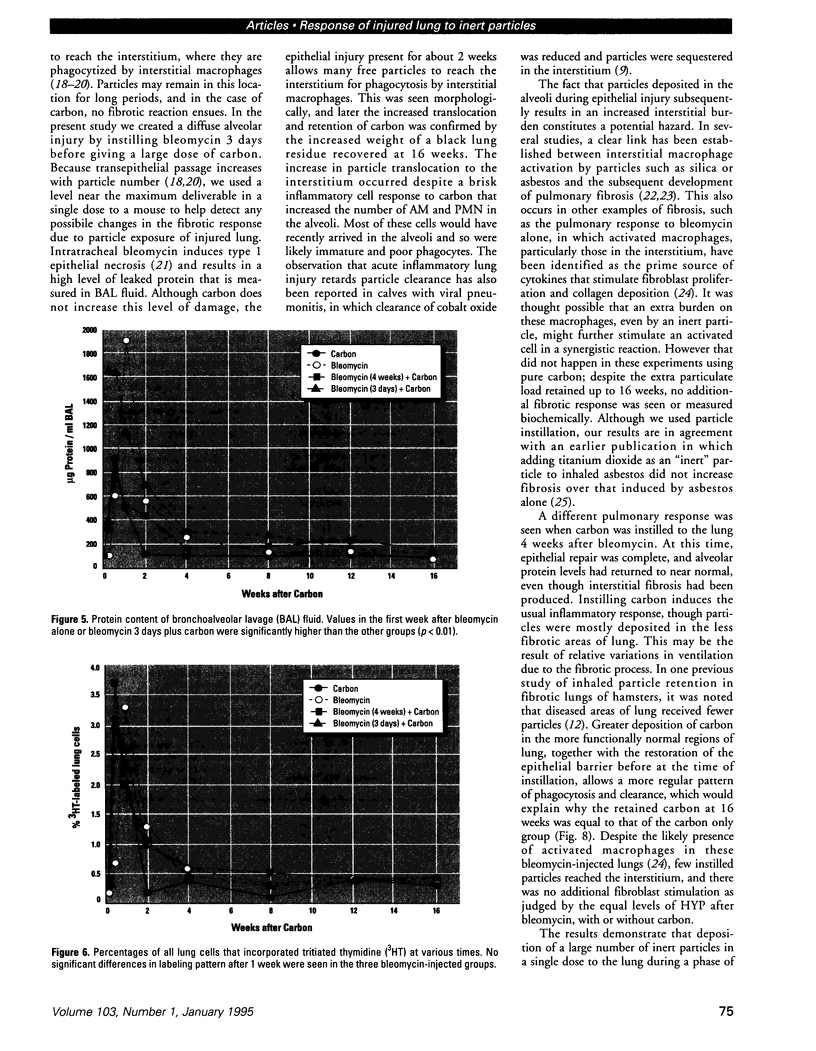
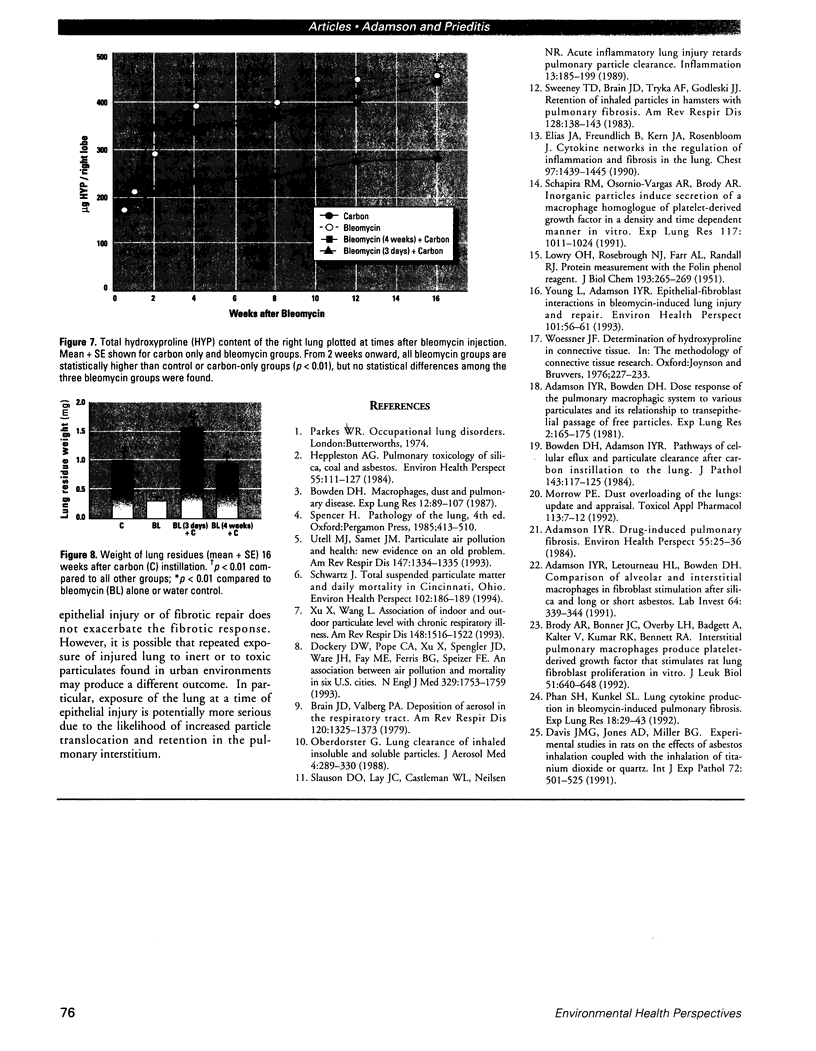
Increased respiratory disease and daily mortality rates are associated with higher levels of fine particulate air pollutants. We examined the possibility that deposition of even inert particles to previously injured lungs may accentuate pulmonary damage by investigating how the lung handles small carbon particles delivered during acute injury or during fibrotic repair. Mice received 2 mg carbon by intratracheal instillation into lungs already showing acute injury, 3 days after bleomycin (BL), or into lungs with fibrosis, 4 weeks after BL. At 3 days after BL, injury to the type I alveolar epithelium resulted in high protein levels in lavage fluid. Instilling carbon at this time induced a large increase in inflammatory cells, though many particles reached the interstitium, and a high proportion was retained up to 16 weeks later. However, fibrosis in these mice was equal to that found after BL alone. In the mice that received carbon 4 weeks after bleomycin, fibrotic repair had already occurred, and the epithelial surface was restored before particle instillation. After carbon, the subsequent inflammatory reaction cleared most particles, little reached the interstitium, and carbon retained at 16 weeks was not different from that in the carbon-only group. Instilling particles into fibrotic lung did not induce additional fibroblast growth or collagen production. The results indicate that instillation of fine particulates to the alveoli at a time of epithelial injury results in increased translocation to the interstitium. However, deposition of pure carbon into injured lungs does not further stimulate an ongoing fibrotic process, although it alters the patterns of particle deposition and retention in the lung.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Dose response of the pulmonary macrophagic system to various particulates and its relationship to transepithelial passage of free particles. Exp Lung Res. 1981 Aug;2(3):165–175. doi: 10.3109/01902148109052312. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y. Drug-induced pulmonary fibrosis. Environ Health Perspect. 1984 Apr;55:25–36. doi: 10.1289/ehp.845525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Letourneau H. L., Bowden D. H. Comparison of alveolar and interstitial macrophages in fibroblast stimulation after silica and long or short asbestos. Lab Invest. 1991 Mar;64(3):339–344. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Pathways of cellular efflux and particulate clearance after carbon instillation to the lung. J Pathol. 1984 Jun;143(2):117–125. doi: 10.1002/path.1711430206. [DOI] [PubMed] [Google Scholar]
- Bowden D. H. Macrophages, dust, and pulmonary diseases. Exp Lung Res. 1987;12(2):89–107. doi: 10.3109/01902148709062834. [DOI] [PubMed] [Google Scholar]
- Brain J. D., Valberg P. A. Deposition of aerosol in the respiratory tract. Am Rev Respir Dis. 1979 Dec;120(6):1325–1373. doi: 10.1164/arrd.1979.120.6.1325. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Bonner J. C., Overby L. H., Badgett A., Kalter V., Kumar R. K., Bennett R. A. Interstitial pulmonary macrophages produce platelet-derived growth factor that stimulates rat lung fibroblast proliferation in vitro. J Leukoc Biol. 1992 Jun;51(6):640–648. doi: 10.1002/jlb.51.6.640. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Jones A. D., Miller B. G. Experimental studies in rats on the effects of asbestos inhalation coupled with the inhalation of titanium dioxide or quartz. Int J Exp Pathol. 1991 Oct;72(5):501–525. [PMC free article] [PubMed] [Google Scholar]
- Dockery D. W., Pope C. A., 3rd, Xu X., Spengler J. D., Ware J. H., Fay M. E., Ferris B. G., Jr, Speizer F. E. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993 Dec 9;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Freundlich B., Kern J. A., Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990 Jun;97(6):1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G. Pulmonary toxicology of silica, coal and asbestos. Environ Health Perspect. 1984 Apr;55:111–127. doi: 10.1289/ehp.8455111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morrow P. E. Dust overloading of the lungs: update and appraisal. Toxicol Appl Pharmacol. 1992 Mar;113(1):1–12. doi: 10.1016/0041-008x(92)90002-a. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Kunkel S. L. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992 Jan-Mar;18(1):29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Schapira R. M., Osornio-Vargas A. R., Brody A. R. Inorganic particles induce secretion of a macrophage homologue of platelet-derived growth factor in a density-and time-dependent manner in vitro. Exp Lung Res. 1991 Nov-Dec;17(6):1011–1024. doi: 10.3109/01902149109064332. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Total suspended particulate matter and daily mortality in Cincinnati, Ohio. Environ Health Perspect. 1994 Feb;102(2):186–189. doi: 10.1289/ehp.94102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauson D. O., Lay J. C., Castleman W. L., Neilsen N. R. Acute inflammatory lung injury retards pulmonary particle clearance. Inflammation. 1989 Apr;13(2):185–199. doi: 10.1007/BF00924789. [DOI] [PubMed] [Google Scholar]
- Sweeney T. D., Brain J. D., Tryka A. F., Godleski J. J. Retention of inhaled particles in hamsters with pulmonary fibrosis. Am Rev Respir Dis. 1983 Jul;128(1):138–143. doi: 10.1164/arrd.1983.128.1.138. [DOI] [PubMed] [Google Scholar]
- Utell M. J., Samet J. M. Particulate air pollution and health. New evidence on an old problem. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1334–1335. doi: 10.1164/ajrccm/147.6_Pt_1.1334. [DOI] [PubMed] [Google Scholar]
- Xu X., Wang L. Association of indoor and outdoor particulate level with chronic respiratory illness. Am Rev Respir Dis. 1993 Dec;148(6 Pt 1):1516–1522. doi: 10.1164/ajrccm/148.6_Pt_1.1516. [DOI] [PubMed] [Google Scholar]
- Young L., Adamson I. Y. Epithelial-fibroblast interactions in bleomycin-induced lung injury and repair. Environ Health Perspect. 1993 Apr 22;101(1):56–61. doi: 10.1289/ehp.9310156. [DOI] [PMC free article] [PubMed] [Google Scholar]