Abstract
Human neutrophil peptide (HNP) defensins were studied to determine their potential effects on adaptive mucosal immunity. Intranasal delivery of HNPs plus ovalbumin (OVA) enhanced OVA-specific serum IgG antibody (Ab) responses. However, OVA-specific IgA Abs were not induced in mucosal secretions or in serum. CD4+ T cells of intranasally immunized mice displayed higher OVA-specific proliferative responses and elevated production of interferon γ, interleukin (IL) 5, IL-6, and IL-10 when compared with control groups receiving OVA alone. In vitro, HNPs also enhanced both proliferative responses and T helper (Th) cytokine secretion profiles of CD3ɛ-stimulated spleen- and Peyer’s patch-derived naive CD4+ T cells. HNPs modulated the expression of costimulatory molecules by lipopolysaccharide- or CD3ɛ-stimulated splenic and Peyer’s patch B or T cell populations, respectively. These studies show that defensins enhance systemic IgG, but not IgA, Ab responses through help provided by CD4+ Th1- and Th2-type cytokines and foster B and T cell interactions to link innate immunity with the adaptive immune system.
Keywords: innate immunity, mucosal immunity, T helper, B7-CD28, CD40-CD40L
Mammals are protected against pathogens by innate and acquired immune mechanisms. Though the epidermis and the mucosal epithelium provide physical protection, innate effector molecules also are required to protect against infections. Among such molecules are defensins, a family of antimicrobial peptides. There are two types of mammalian defensins, alpha and beta, which differ in their distribution and connection of six cysteine residues. Classical or alpha defensins are found in most mammalian neutrophil granules, macrophages, and mucosal crypt and Paneth cells (1–6). Beta defensins are found in bovine neutrophils, tracheal epithelium, avian leukocytes, and human plasma (7–9). Defensins are cytotoxic peptides and presumably permeate the membranes of a wide range of organisms, including Gram-negative and Gram-positive bacteria (3, 10), fungi (11), parasites (12), viruses (13), and mycobacteria (14). High (μM) concentrations of defensins are toxic to mammalian cells (15) and also may be involved in the antibody (Ab)-dependent lysis of tumor cells (16). Lower (nM) concentrations have been shown to be mitogenic for murine epithelial cells and fibroblasts (17). Two human defensins, human neutrophil peptide (HNP) 1 and HNP-2, have been shown to be chemotactic for murine and human T cells (18), monocytes, and polymorphonuclear leukocytes (19). Even though murine neutrophils do not express defensins (20), the >60% homology and 40% identity of HNP-1, -2, and -3 with murine crypt cell defensins (i.e., cryptins) (1, 21, 22) and the high quantity of HNPs that can be purified from human peripheral blood make these proteins the most efficient way to study the effects of the alpha defensin superfamily on acquired host immunity. Furthermore, the residues important for the structure and function of these proteins are conserved (22); it is rational to suggest that alpha defensins have similar structure and function. For instance, the neutrophil is one of the first leukocytes present during an inflammatory or infectious process in the periphery and mucosa. Correspondingly, crypt and Paneth cells are among the first cells to encounter foreign antigen (Ag) in the mucosa. It is indeed likely that crypt and neutrophil defensins released during these encounters affect the lymphocytes present in these environments in a similar fashion. In this regard, it has been demonstrated that HNPs intraperitally administered with keyhole limpet hemocyanin (KLH) enhanced KLH-specific peripheral immune responses of mice (J.J.O., unpublished observations).
Defensins are expressed by mammalian epithelium during chronic and acute infections without causing host cell damage (22–24). We hypothesized that in addition to defensins’ antimicrobial functions, the presence of these multifunctional peptides in the mucosa may trigger adaptive immune responses to foreign Ag. Our studies provide evidence that the presence of defensins in the mucosa can promote acquired systemic immune responses without significantly inducing mucosal immunity.
MATERIALS AND METHODS
Defensins.
HNP-1, -2, and -3 were isolated from the granules of polymorphonuclear leukocytes from normal donors as reported (18). Amino acid sequence analysis of the preparation revealed the presence of three related sequences: HNP-1, which has Ala as the N-terminal residue (50%); HNP-3, which has Asp as the N-terminal residue (20%), and HNP-2, which is shorter than HNP-1 and HNP-3 by one N-terminal residue (30%). No other sequences were detected, and the results of amino acid analysis corresponded with the amino acid composition of these defensins, suggesting that the preparation was >95% pure. No protein contaminants were detected by matrix-assisted laser desorption ionization and time-of-flight MS. The endotoxin level of the HNP formulation was below levels quantifiable by the amebocyte lysate assay (Associates of Cape Cod).
Mice, Immunizations, and Sample Collection.
Female, 6- to 8-week-old C57BL/6 mice were procured from Charles River Breeding Laboratories. After anesthesia, mice were immunized intranasally on days 0, 7, and 14 with 50 μg of ovalbumin (OVA) (Sigma) in the presence or absence of HNPs in 10 μl of PBS, pH 7.4. Nasal, saliva, vaginal, and fecal secretions and serum samples were collected as described (25). One week after the last immunization, mice were sacrificed to quantify the OVA-specific B and T cell responses.
Total and OVA-Specific Ab Detection by ELISA.
To obtain OVA-specific Ab titers, flexible Falcon ELISA plates (Fisher Scientific) were coated with 1 mg/ml of OVA. Similarly, 0.5 μg/ml of goat anti-mouse Ig (H+L) polyclonal Ab (Southern Biotechnology Associates) in carbonate-bicarbonate pH 9.5 were added to ELISA plates to determine total Ab levels. The titers of IgM, IgG, or IgA Abs were determined by the addition of a 1:3,000 dilution of horseradish peroxidase (HRP)-conjugated, goat anti-mouse-α, -γ, or -μ heavy chain-specific polyclonal Abs (Southern Biotechnology Associates) in PBS containing 1% BSA and 0.05% Tween (B-PBS-T). IgG subclasses and IgE Ab titers were assessed with biotin-conjugated rat anti-mouse-γ1 (G1–7.3 at 12.5 ng/ml), -γ2a (R19–15 at 125 ng/ml), -γ2b (R12–3 at 12.5 ng/ml), -γ3 (R40–82 at 50 ng/ml), and -ɛ (G1–7.3; 1.25 μg/ml) (PharMingen) mAbs (25). HRP-antibiotin mAb (0.5 μg/ml) (Vector Laboratories) in B-PBS-T were added to plates containing samples of IgG subclass Ab. For IgE isotype detection, 500 ng/ml of streptavidin-conjugated poly-HRP80 (Research Diagnostics, Flanders, NJ) in poly-HRP diluent (Research Diagnostics) was added to detection wells. The color reaction for ELISAs was developed by adding 1.1 mM 2,2′-azino-bis(3)-ethylbenz-thiazoline-6-sulfonic acid (Sigma) in 0.1 M citrate-phosphate buffer (pH 4.2) containing 0.01% H2O2. Endpoint titers were expressed as the reciprocal log2 of the highest dilution, which gave an OD at 415 nm of ≥0.1 above negative controls (26).
Cell Isolation Procedures.
Single cell suspensions of spleen, salivary glands, Peyer’s patches, and mesenteric, cervical, and ileal lymph nodes were prepared by aseptically removing tissues and passing them through a sterile wire screen. A cell suspension prepared from the nasal-associated lymphoreticular tissue in the nasal passage of mice was removed by scraping (27). Lower respiratory tract tissues were excised, minced, and further disrupted by incubation at 37°C with stirring in 3 mg/ml of collagenase type IV (Sigma) in RPMI medium 1640 (27). Lamina propria lymphocytes from the small intestine were isolated as described (27). Lymphocytes used ex vivo were maintained in complete medium, which consisted of RPMI medium 1640 supplemented with 10 ml/liter of nonessential amino acids (Mediatech, Washington, DC), 1 mM sodium pyruvate (Sigma), 10 mM Hepes, 100 units/ml of penicillin, 100 μg/ml of streptomycin (Mediatech), 40 μg/ml gentamycin (Elkins-Sinn, Cherry Hill, NJ), 50 μM 2-mercaptoethanol (Sigma), and 10% fetal calf serum (Atlanta Biologicals, Norcross, GA). T cells were fractionated by passing the single cell suspensions over nylon wool columns. CD4+ T cells were enriched (>98% purity) by using Mouse CD4 Cellect Plus columns according to manufacturer’s protocols (Biotex Laboratories, Edmonton, Canada).
Enzyme-Linked Immunospot (ELISPOT) Assays.
An ELISPOT assay was used to detect total Ig isotypes or OVA-specific Ab-forming cells (AFCs) (26). In brief, 96-well Millititer hemagglutinin nitrocellulose-based plates (Millipore) were coated with 1 mg/ml of OVA in PBS, PBS only (negative control), or 0.5 μg/ml of goat anti-mouse Ig (H+L) Ab (Southern Biotechnology Associates). Individual AFCs were detected with HRP-labeled goat anti-mouse-α, -μ, or -γ heavy chain-specific Abs (1 μg/ml; Southern Biotechnology Associates), visualized by adding 3-amino-9-ethylcarbazole buffer (Moss, Pasadena, MD).
OVA-Specific and HNP-Mediated T Cell Responses.
CD4+ T cells from immunized mice were cultured at a density of 5 × 106 cells/ml with T cell-depleted and -irradiated (3,000 rads) splenic feeder cells (1 × 106 cells/ml) in complete medium containing OVA. CD4+ T cells from unimmunized mice were cultured with 500 ng/ml of anti-mouse CD3ɛ mAb (145–2C11; PharMingen) in the absence or presence of HNPs. T cells from nonimmunized mice were stimulated with 10 μg/ml of anti-mouse CD3ɛ mAb or BSA as positive or negative controls, respectively. To ascertain Ag-specific or HNP-mediated proliferative responses after incubation for 3 days, the cell cultures were pulsed with 0.5 μCi of methyl-3H-thymidine (Amersham Pharmacia) per well for the final 18 hr of culture. Cells were harvested onto glass microfiber filter paper (Whatman), and radioactivity was determined by liquid scintillation counting.
Cytokine ELISA.
For the assessment of cytokine production, the supernatants from CD4+ T cell cultures were harvested after 5 days of incubation. Control wells consisted of cells only or cells cultured with BSA or 1 μg/ml of Con A (Sigma). Supernatants from the treated nonimmunized CD4+ T cell cultures were analyzed after 3 days. Cytokine levels in culture supernatants were determined by ELISA as described (25). The cytokine ELISA assays were capable of detecting 15 pg/ml of interferon (IFN) γ, 5 pg/ml of interleukin (IL) 2, IL-4, and IL-5, 10 pg/ml of IL-6 and 20 pg/ml of IL-10.
In Vitro Effects of HNPs.
Ninety-six-well, round bottom-plates were coated with 0, 100, or 500 ng/ml of anti-mouse-CD3ɛ mAb in carbonate-bicarbonate buffer, pH 9.4. Spleen- or Peyer’s patch-derived lymphocytes from naive mice were added at a density of 5 × 106 cells/ml in complete medium containing HNPs. Lymphocytes were incubated with 500 ng/ml of lipopolysaccharide (LPS) from Salmonella minnesota Re595 (Calbiochem) in the presence or absence of HNPs in polypropylene conical tubes. Lymphocytes were incubated with optimal doses of LPS (10 μg/ml), Con A (5 μg/ml), or anti-mouse CD3ɛ mAb (10 μg/ml coated plates) as positive controls or alone as negative controls. After incubation for 2 days, cells were stained with rat anti-mouse-B220, -IL2R, -CD3ɛ, -CD28, -CD40, -CD40L, -CD80, -D86, -CTLA4, and/or -MAC-1 mAbs conjugated to either phosphatidylethanolamine or fluorescein isothiocyanate (PharMingen) and analyzed by flow cytometry.
Statistics.
The data are expressed as the mean ± SEM and compared by using a two-tailed Student’s t test or an unpaired Mann–Whitney U test. The results were analyzed by using the statview II statistical program (Abacus Concepts, Berkeley, CA) for Macintosh computers and were considered statistically significant if P values were less than 0.05. For cytokine levels of samples below the detection limit, levels were recorded as half the lower detection limit (e.g., 5 pg/ml = IL-6) for statistical analysis.
RESULTS
Dose Response and Kinetics of Serum Ab Responses.
Because intranasal immunization has been shown to be an effective way to induce mucosal and systemic Ab responses to protein Ags (28), we administered OVA with increasing concentrations of HNPs in a similar manner. Intranasal immunization with OVA alone elicited low OVA-specific serum IgM and IgG Ab responses. Mice receiving OVA plus 1.0 or 5.0 μg of HNPs displayed significantly higher (P < 0.01) serum titers of OVA-specific IgG and IgM Ab responses. Lower doses of HNPs also enhanced IgG anti-OVA Abs. Mucosal secretion samples of mice immunized with HNPs did not display any significant increases of OVA-specific Ab titers. Hence, we used 1 μg of HNPs per intranasal dose in subsequent experiments.
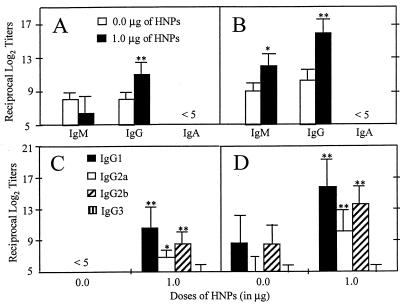
We next assessed the titers of serum anti-OVA Abs of mice intranasally immunized with OVA and HNPs at weekly intervals. No OVA-specific Abs other than IgM were detectable by day 7, and the Ab titers of mice immunized with OVA plus HNPs were comparable to those of control groups (i.e., OVA only or PBS only). However, both IgM and IgG Ab titers were elevated after two intranasal immunizations (Fig. 1A). By day 21, Ag-specific IgM in both groups increased but the highest serum Ab responses to OVA were of the IgG isotype (Fig. 1B). OVA-specific serum IgA or IgE Abs were not detected during the immunization regimen (Fig. 1 and data not shown). The OVA-specific IgG response was further characterized by using IgG subclass analysis by ELISA. Administration of HNPs + OVA resulted in increased serum IgG1, followed by IgG2b and IgG2a anti-OVA Abs by day 14 (Fig. 1C). As expected, the third immunization enhanced the OVA-specific IgG1 > IgG2b > IgG2a serum Ab responses (Fig. 1D). The intranasal administration of HNPs + OVA did not influence IgG3 anti-OVA Ab titers (Fig. 1 C and D).
Figure 1.
Kinetics of serum OVA-specific IgA, IgM, and IgG and IgG subclass Ab responses. After anesthesia, groups of C57BL/6 mice were intranasally immunized three times on days 0, 7, and 14 with 50 μg of OVA and 0.0 or 1.0 μg of HNPs in 10 μl of PBS. The data presented are the mean Ab titers ± SEM of these experiments. Experimental groups consisted of five mice, and studies were repeated three times. The data distribution of OVA-specific IgA, IgM, and IgG serum Abs collected on day 14 (A) and day 21 (B) was determined by ELISA. The profile of Ag-specific IgG subclasses of serum samples taken on day 14 (C) and day 21 (D) also was determined by ELISA. The data presented are the mean Ab titer ± SEM. Asterisks indicate statistically significant differences (∗, P < 0.05; ∗∗, P < 0.01) relative to Ab titers of mice immunized with OVA alone.
HNP Effects on Mucosal Ab Responses.
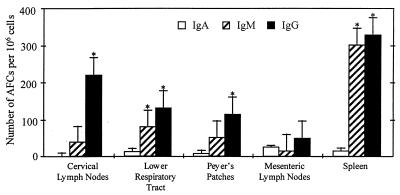
Intranasal immunization with HNPs did not induce Ag-specific IgA Ab responses in nasal wash or saliva, and there was no detectable increase in IgM anti-OVA Abs during the immunization schedule. In addition, there was no increase in IgA anti-OVA Ab responses in vaginal washes or fecal samples. A total of three intranasal doses of HNPs with OVA at weekly intervals slightly enhanced OVA-specific IgG titers in vaginal wash samples or fecal extracts compared with mice receiving OVA alone. Both ELISA and ELISPOT analysis did not reveal any statistically significant change in the level of total Igs or AFCs present in these mucosal secretions or in mucosal-inductive or effector sites (data not shown). OVA-specific ELISPOT assays were performed on cells from mucosal and systemic tissues to determine whether the low mucosal anti-OVA Ab responses observed arose from mucosal inductive and/or effector sites. Spleen and cervical lymph node lymphocytes showed significant increases in Ag-specific IgG and IgM AFCs, whereas lower respiratory tract-, Peyer’s patch-, and mesenteric lymph node-derived cells displayed only low to moderate increases in anti-OVA IgG and IgM AFCs (Fig. 2). There were either minimal or undetectable increases in anti-OVA AFCs (Fig. 2) from salivary gland, mesenteric lymph node, and gastrointestinal lamina propria tissues (data not shown).
Figure 2.
Numbers of Ag-specific AFCs in peripheral and mucosal tissues after intranasal immunization with HNPs and OVA. After anesthesia, groups of C57BL/6 mice were intranasally immunized on days 0, 7, and 14 with 50 μg of OVA and 0.0 or 1.0 μg of HNPs in 10 μl of PBS. Experimental groups consisted of five mice, and studies were repeated three times. OVA-specific IgA, IgM, and IgG AFCs present in cervical lymph nodes, lower respiratory tract and associated lymphoid tissues, Peyer’s patches, mesenteric lymph nodes, and spleens were determined by ELISPOT analysis. The data presented are the mean AFCs ± SEM 1 week after the last immunization of these experiments. Asterisks indicate statistically significant differences (∗, P < 0.05; ∗∗, P < 0.01) relative to Ab titers of mice immunized with OVA alone.
Proliferative Responses and Cytokine Profiles of OVA-Specific T Cells.
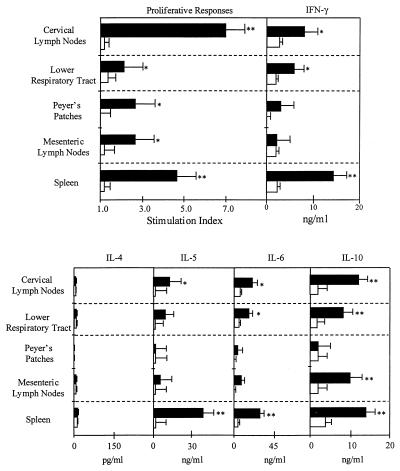
Because HNPs promoted systemic Ab responses but did not induce significant mucosal Ab responses after intranasal immunization, we asked whether OVA-specific T cells generated by our regimen would display different T helper (Th) cell cytokine profiles depending on their source (e.g., mucosal or splenic tissues). The CD4+ T cells isolated from these immune compartments showed marked increases in OVA-specific proliferative responses (Fig. 3), with cervical lymph node- and spleen-derived CD4+ T cells from mice that received defensins showing the highest increases in OVA-specific proliferative responses. Correspondingly, OVA-specific CD4+ T cells from cervical lymph node and splenic tissues produced greater increases in IFN-γ, IL-5, IL-6, and IL-10 in culture than did CD4+ T cells isolated from the lower respiratory tract, mesenteric lymph nodes, or Peyer’s patches (Fig. 3). However, IL-4 was not significantly induced in any of the immune compartments studied (Fig. 3).
Figure 3.
OVA-specific proliferation and induction of Th1- and Th2-type cytokine secretion by spleen- and mucosa-derived CD4+ T cells. After anesthesia, groups of C57BL/6 mice were intranasally immunized on days 0, 7, and 14 with 50 μg of OVA and 0.0 (empty boxes) or 1.0 μg (filled boxes) of HNPs in 10 μl of PBS. One week after the last immunization, lower respiratory tract, Peyer’s patches, cervical, mesenteric, and spleen lymphoid tissue-derived CD4+ T cells were purified and cultured at a density of 5 × 106 cells/ml with 500 μg/ml of OVA for 3 days with T-cell-depleted and -irradiated splenic feeder cells (1 × 106 cells/ml) in complete medium. Experimental groups consisted of five mice, and studies were repeated three times. Proliferation was measured by 3H-thymidine incorporation. The stimulation index corresponds to the cpm of cell cultures containing OVA divided by the cpm of cultures with no additions. The data presented are the mean stimulation index ± SEM. ∗ indicate statistically significant differences (∗, P < 0.05; ∗∗, P < 0.01) relative to the stimulation index of mice immunized with OVA alone. Cytokine protein production of these cultured supernatants was determined by ELISA. The data presented are the mean cytokine levels (pg/ml) ± SEM in each group. Asterisks indicate statistically significant differences (∗, P < 0.05; ∗∗, P < 0.01) relative to cytokine levels of mice immunized with OVA alone.
Effects of HNPs on CD3ɛ-Stimulated CD4+ T Cells.
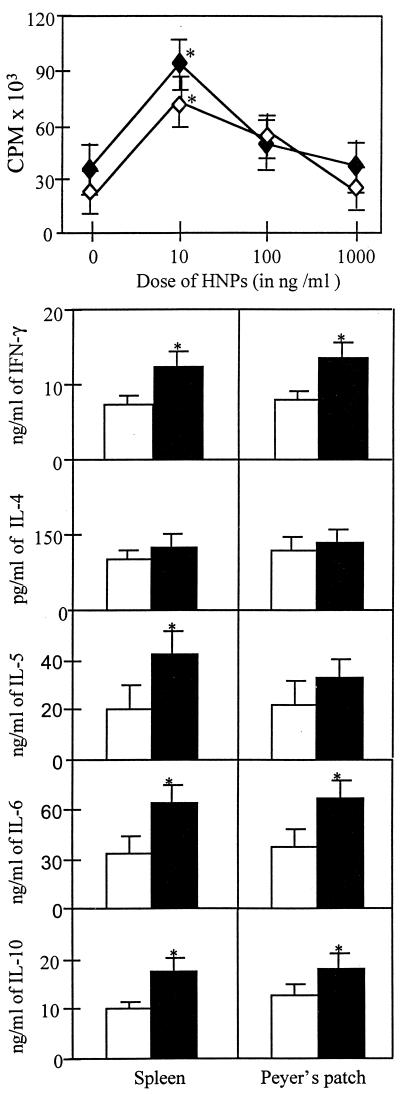
To better elucidate the effects of HNPs on T cell responses, we assessed the potential of HNPs to act as mitogens to affect proliferative responses and cytokine secretion by naive CD4+ T lymphocytes. HNPs alone were not mitogenic; however, defensins enhanced the proliferation of CD3ɛ-stimulated spleen and Peyer’s patch CD4+ T lymphocytes in a dose-dependent manner (Fig. 4). Concentrations of defensins ≥1 μg/ml resulted in either suppression or reduction of proliferative responses (Fig. 4); notably, the viability of cultures incubated (>6 hr) with >1 μg/ml of HNPs was reduced by >50% (data not shown). In vitro, HNPs significantly increased (P < 0.05) the levels of IFN-γ, IL-6, and IL-10 without drastically increasing the secretion of IL-5 cytokines from both Peyer’s patch- and spleen-derived CD3ɛ-activated CD4+ T cells (Fig. 4). Most notably, HNPs did not enhance the secretion of IL-4 (Fig. 4). The proliferative responses and cytokine induction exacted by HNPs on CD3ɛ-stimulated naive CD4+ T cells correlated with the Ag-specific responses observed ex vivo.
Figure 4.
Anti-CD3ɛ and HNP-mediated proliferative responses and cytokine secretion by naive CD4+ T lymphocytes. Spleen- (⧫) or Peyer’s patch-derived (◊) cells were isolated from naive mice and stimulated in vitro in rat anti-mouse CD3ɛ-coated 96-well plates with 0, 10, 100, or 1,000 ng/ml of HNPs. Cytokine protein production of cultured supernatants containing a suboptimal dose of anti-mouse CD3 mAb and 0 (□) or 10 ng/ml (■) of HNPs was determined by ELISA. Experimental studies were repeated three times, and the data presented are the mean proliferation ± SEM measured by 3H-thymidine incorporation and illustrated as cpm or the mean cytokine levels ± SEM in each group. Asterisks indicate the statistically significant differences (P < 0.05) relative to the cpm of or cytokine levels of CD4+ T cells incubated without HNPs.
Defensins increased the numbers of CD3+ IL2R+ cells as much as 125% in splenic and 48% in Peyer’s patch lymphocytes (Table 1). HNPs also up-regulated CD28 expression by T cells from the spleen (65% to 98% increases) as well as Peyer’s patches (29% to 57% increases) but decreased the CTLA-4 expression by Peyer’s patch T cells as much as 36% (Table 1). Finally, we observed a negligible though consistent ∼10% decrease of splenic CD3+ CD40L+ cells and a 34–42% decrease of CD40L expression by Peyer’s patch T cells (Table 1).
Table 1.
Regulation of costimulatory molecule expression on CD3ɛ-activated T cells by defensins
| Costimulatory molecule | Splenic T cells
|
Peyer’s patch T cells
|
||
|---|---|---|---|---|
| 10 ng/ml | 100 ng/ml | 10 ng/ml | 100 ng/ml | |
| IL2R | 73 ± 5.0%* | 125 ± 12.3%* | 22 ± 1.3% | 48 ± 3.4%* |
| CD28 | 65 ± 3.9%* | 98 ± 9.7%* | 29 ± 2.2% | 57 ± 2.7%* |
| CTLA4 | (12 ± 0.8%) | (27 ± 1.5%) | (15 ± 0.9%) | (36 ± 2.4%) |
| CD40L | (4 ± 0.3%) | (13 ± 0.7%) | (34 ± 1.7%) | (42 ± 3.1%)* |
CD3ɛ-activated splenic and Peyer’s patch T cells from naive mice were incubated with 0, 10, and 100 ng/ml of HNPs. The percent increase (or decrease) of the costimulatory molecule expression on CD3ɛ-activated T cells was calculated as the percent of double positive CD3+ and IL2R+, CD28+, CTLA-4, or CD40L+ cells in cultures containing HNPs minus the percent gated of double positive cells in cultures without HNPs divided by the latter. Studies were repeated three times, and the data presented are the mean percent change ± SEM of these experiments.
Statistically significant differences (P < 0.05) relative to cultures without HNPs.
Effects of HNPs on LPS-Stimulated B Cells.
To better understand the effects that HNPs have on B cell responses, we analyzed the potential of HNPs to act as regulatory molecules to affect costimulatory molecule expression by these lymphocytes. Thus, we characterized CD80, CD86, and CD40 expression on LPS-stimulated naive murine lymphocytes with increasing concentrations of HNPs. Defensins increased the LPS-induced expression of CD40 on B220+ cells (Table 2). The HNP-mediated induction of CD40 on splenic and Peyer’s patch B cells was essentially the same, e.g., ≈50% increases with 10 ng/ml or 100 ng/ml of HNPs, respectively (Table 2). However, HNPs + LPS reduced the level of CD86 expression on B cells (Table 2).
Table 2.
Regulation of costimulatory molecule expression on LPS-activated B cells by defensins
| Costimulatory molecule | Splenic B cells
|
Peyer’s patch B cells
|
||
|---|---|---|---|---|
| 10 ng/ml | 100 ng/ml | 10 ng/ml | 100 ng/ml | |
| CD80 | 1 ± 0.1% | (3 ± 0.3%) | 6 ± 1.10% | 13 ± 2.6% |
| CD86 | (47 ± 3.4%)* | (15 ± 1.1%) | (11 ± 0.6%) | (32 ± 1.7%)* |
| CD40 | 51 ± 5.6%* | 103 ± 10.1% | 50 ± 4.8%* | 80 ± 7.2%* |
LPS-stimulated splenic and Peyer’s patch B cells from naive mice were incubated in the presence or absence of HNPs and prepared for FACS analysis. The percent increase or (decrease) in costimulatory molecule expression by LPS-stimulated B cells was calculated as the percent of double positive B220+ and CD80+, CD86+, or CD40+ cells in cultures containing HNPs minus the percent of double positive cells in cultures without HNPs divided by the latter. Studies were repeated three times, and the data presented are the mean percent change ± SEM of these experiments.
Statistically significant differences (P < 0.05) relative to cultures without HNPs.
DISCUSSION
Defensins are chemotactic for T cells and therefore may be capable of activating T cell-dependent immune responses. Hence, the possible role of exogenous defensins in the mucosa to develop adaptive immunity was investigated. The expression of some of the defensin superfamily members on mucosal surfaces and in the serum warranted the use of an intranasal immunization strategy that potentially induces both mucosal and systemic immune responses (28). Our study elucidates the enhancing effects of defensins on immune responses to foreign Ag in vivo. We fully characterized the adaptive immune response promoted by these peptides and found that HNPs significantly increased Ag-specific IgG and IgM Ab levels in the serum. These serum Ab responses were associated with the induction of Ag-specific CD4+ T cell proliferative responses and IFN-γ, IL-5, IL-6, and IL-10 secretion. Our results support the major premise of our initial hypothesis that defensins enhance acquired host responses.
The significant increases in IgG1, the absence of IgE, and the subsequent expression of IgG2b and IgG2a OVA-specific Ab titers most likely can be accounted for by the mixed Th1/Th2-type cytokine help provided by CD4+ T cells as well as by our use of a soluble protein Ag (29–32). We also noted greater proliferative responses and cytokine secretion profiles by CD4+ T cells isolated from the spleen compared with those isolated from Peyer’s patches. These findings suggest that HNPs may act differently on splenic versus Peyer’s patch–derived T cells to induce higher humoral and cellular immune responses in systemic immune compartments.
Our results show that CD3ɛ stimulation and HNPs enhanced proliferative responses and increased the CD3+ IL2R+ population. B7 ligands (CTLA-4 and CD28) are equally expressed by CD4+ and CD8+ T cells and cooperatively regulate T cell adhesion and activation through B7 and T cell Ag receptor stimulation (33). Therefore, we assessed the effects of defensins on CD28, CTLA-4, and CD40L expression by T cells. HNPs increased IL2R and CD28 expression by spleen and Peyer’s patch T cells in a dose-dependent manner. In contrast, CTLA-4 and CD40L expression by CD3ɛ-stimulated T cells was either unchanged or somewhat reduced after incubation with HNPs. Although CD28-B7 interactions have been shown to promote T cell activation (34), conflicting reports suggest that CTLA-4-B7 costimulatory signals may lead to either suppression and tolerance (35) or optimal T cell activation and differentiation (36). Hence, there is no apparent explanation, at this time, for the modest down-regulation of CTLA-4 expression by HNP-treated lymphocytes. The differential regulation of CD28 and CD40L (37) may account for the humoral and cellular immune responses detected in vivo.
During a microbial infection, nonpeptide Ags or agents are involved in the generation of immune responses. For example, LPS activates Ag-presenting cells through T cell-independent interactions (38). Therefore we evaluated the potential of defensins to alter the expression of costimulatory molecules induced by LPS. HNPs enhanced the expression of CD40 by spleen- and Peyer’s patch-derived B cells. Although the expression of CD80 was unaffected, HNPs down-regulated the expression of CD86 by LPS-stimulated B cells (Table 2). These effects were more pronounced on Peyer’s patch than on splenic B cells. Taken together, these results suggest that HNPs can modulate B7-CD28 and CD40-CD40L interactions upon Ag stimulation.
Defensins are thought to protect the epithelium from invading microorganisms through their bactericidal activity. It is interesting that these innate peptides initiated significant systemic, but not mucosal, humoral immunity after nasal delivery. Several reasons could have accounted for the low or minimal IgA Ab levels in mucosal secretions. It is possible that the cytokines induced by HNPs do not support the switch to or synthesis of IgA Abs. It is unlikely that HNPs disrupted the cell integrity of the mucosa, which could have allowed nasally administered Ag to be delivered directly to the periphery. In fact, defensins expressed on the surface of mucosal epithelium during infections, at comparable levels used in our assays, did not damage the cell integrity of the host (22–24). Further, histological analysis showed that the nasal passages were unaffected by the defensin concentrations used in our study (data not shown). Even though HNPs are not disruptive to host cell integrity, defensins may cause subtle changes in epithelial cells and their tight junctions to allow extracellular HNPs with foreign Ag to be rapidly absorbed by the mucosa and thus gain access to peripheral lymphoid tissues to increase systemic immune responses. Additionally, the regulatory effects of defensins on costimulatory molecule expression by lymphocytes also could induce differential host immunity.
Classical inducers of S-IgA, in contrast to defensins, up-regulate CD28, CD80, and CD86 molecules (39, 40). HNPs may not induce the initial B and T cell interactions necessary for the switch to IgA+ B cells and/or the costimulatory signals essential for IgA Ab synthesis in the mucosa. Alpha defensins, produced by mucosal crypt and Paneth cells, are present in both mice and humans. We have shown that HNP-alpha defensins enhanced IL2R and CD28 expression by splenic T cells more dramatically than by Peyer’s patch-derived T lymphocytes, which suggests a tissue-specific effect by these classical defensins. Organ-specific immune regulation by host factors is not uncommon. Neuropeptides regulate Ig production and mitogen-induced lymphocyte proliferation in a tissue-specific manner (41, 42). Substance P was shown to increase IgA synthesis by 70% from splenocytes compared with the 300% increases observed from Peyer’s patch-derived lymphocytes (43).
We have shown that defensins provide signals to bridge innate and adaptive immunity. It has been shown that defensins are up-regulated in the mucosal epithelium after microbial infection (22–24). The previously described bactericidal effects of defensins are thought to contribute to the protection of the mucosa. In this study, we have revealed the role defensins play in enhancing host immunity. Potentially, these characteristics would allow crypt and Paneth cell defensins to contain pathogens and commensals in mucosal compartments without invoking brisk mucosal immune responses whenever a foreign substance is encountered in the mucosa. Correspondingly, these mechanisms would permit defensins to markedly enhance Ag-specific host responses to microbes that enter the sterile peripheral environment. Although our results contribute to understanding of the role of defensins in adaptive immunity, additional studies will be needed to resolve the precise contributions defensins make toward the generation of immune responses.
Acknowledgments
We thank Dr. Kimberly McGhee for preparation of the written text of this manuscript. The content of this manuscript benefited from discussions with members of the University of Alabama at Birmingham Immunobiology Vaccine Center. This work was supported by National Institutes of Health Grants AI 18958, AI 43197, and DK 44240 and the United Negro College Fund–Merck Postdoctoral Science Research Fellowship.
ABBREVIATIONS
- Ab
antibody
- AFC
Ab-forming cell
- Ag
antigen
- ELISPOT
enzyme-linked immunospot
- HNP
human neutrophil peptide
- HRP
horseradish peroxidase
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- OVA
ovalbumin
- Th
T helper
References
- 1.Eisenhauer P B, Harwig S S, Lehrer R I. Infect Immun. 1992;60:3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann J, Church J A, Lehrer R I. Am J Med Sci. 1986;291:334–341. doi: 10.1097/00000441-198605000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Selsted M E, Szklarek D, Lehrer R I. Infect Immun. 1984;45:150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harwig S S, Park A S, Lehrer R I. Blood. 1992;79:1532–1537. [PubMed] [Google Scholar]
- 5.Porter E M, Vandam E, Valore E V, Ganz T. Infect Immun. 1997;65:2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter E M, Liu L, Oren A, Anton P A, Ganz T. Infect Immun. 1997;65:2389–2395. doi: 10.1128/iai.65.6.2389-2395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selsted M E, Tang Y Q, Morris W L, McGuire P A, Novotny M J, Smith W, Henschen A H, Cullor J S. J Biol Chem. 1993;268:6641–6648. [PubMed] [Google Scholar]
- 8.Harwig S S, Swiderek K M, Kokryakov V N, Tan L, Lee T D, Panyutich E A, Aleshina G M, Shamova O V, Lehrer R I. FEBS Lett. 1994;342:281–285. doi: 10.1016/0014-5793(94)80517-2. [DOI] [PubMed] [Google Scholar]
- 9.Bensch K W, Raida M, Magert H J, Schulz-Knappe P, Forssmann W G. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 10.Ganz T, Selsted M E, Szklarek D, Harwig S S, Daher K, Bainton D F, Lehrer R I. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selsted M E, Szklarek D, Ganz T, Lehrer R I. Infect Immun. 1985;49:202–206. doi: 10.1128/iai.49.1.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aley S B, Zimmerman M, Hetsko M, Selsted M E, Gillin F D. Infect Immun. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehrer R I, Daher K, Ganz T, Selsted M E. J Virol. 1985;54:467–472. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogata K, Linzer B A, Zuberi R I, Ganz T, Lehrer R I, Catanzaro A. Infect Immun. 1992;60:4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Wetering S, Mannesse-Lazeroms S P, Dijkman J H, Hiemstra P S. J Leukocyte Biol. 1997;62:217–226. doi: 10.1002/jlb.62.2.217. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein A, Ganz T, Selsted M E, Lehrer R I. Blood. 1986;68:1407–1410. [PubMed] [Google Scholar]
- 17.Murphy C J, Foster B A, Mannis M J, Selsted M E, Reid T W. J Cell Physiol. 1993;155:408–413. doi: 10.1002/jcp.1041550223. [DOI] [PubMed] [Google Scholar]
- 18.Chertov O, Michiel D F, Xu L, Wang J M, Tani K, Murphy W J, Longo D L, Taub D D, Oppenheim J J. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 19.Territo M C, Ganz T, Selsted M E, Lehrer R I. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer P B, Lehrer R I. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huttner K M, Selsted M E, Ouellette A J. Genomics. 1994;19:448–453. doi: 10.1006/geno.1994.1093. [DOI] [PubMed] [Google Scholar]
- 22.Jones D E, Bevins C L. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 23.Stolzenberg E D, Anderson G M, Ackermann M R, Whitlock R H, Zasloff M. Proc Natl Acad Sci USA. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darmoul D, Brown D, Selsted M E, Ouellette A J. Am J Physiol. 1997;272:G197–G206. doi: 10.1152/ajpgi.1997.272.1.G197. [DOI] [PubMed] [Google Scholar]
- 25.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 26.Jackson R J, Fujihashi K, Xu-Amano J, Kiyono H, Elson C O, McGhee J R. Infect Immun. 1993;61:4272–4279. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staats H F, Nichols W G, Palker T J. J Immunol. 1996;157:462–472. [PubMed] [Google Scholar]
- 29.Scott M G, Fleischman J B. J Immunol. 1982;128:2622–2628. [PubMed] [Google Scholar]
- 30.Stevens T L, Bossie A, Sanders V M, Fernandez-Botran R, Coffman R L, Mosmann T R, Vitetta E S. Nature (London) 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 31.Snapper C M, Finkelman F D, Paul W E. J Exp Med. 1988;167:183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkelman F D, Katona I M, Urban J F, Jr, Holmes J, Ohara J, Tung A S, Sample J V, Paul W E. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 33.Azuma M, Ito D, Yagita H, Okumura K, Phillips J H, Lanier L L, Somoza C. Nature (London) 1993;366:76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 34.Freedman A S, Freeman G J, Rhynhart K T, Nadler L M. Cell Immunol. 1991;137:429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 35.Walunas T L, Bluestone J A. J Immunol. 1998;160:3855–3860. [PubMed] [Google Scholar]
- 36.Lu P, Zhou X, Chen S J, Moorman M, Morris S C, Finkelman F D, Linsley P, Urban J F, Gause W C. J Exp Med. 1994;180:693–698. doi: 10.1084/jem.180.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIntyre T M, Kehry M R, Snapper C M. J Immunol. 1995;154:3156–3161. [PubMed] [Google Scholar]
- 38.Morrison D C, Ryan J L. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 39.Cong Y Z, Weaver C T, Elson C O. J Immunol. 1997;159:5301–5308. [PubMed] [Google Scholar]
- 40.Gärdby E, Lane P, Lycke N Y. J Immunol. 1998;161:49–59. [PubMed] [Google Scholar]
- 41.Ottaway C A, Greenberg G R. J Immunol. 1984;132:417–423. [PubMed] [Google Scholar]
- 42.Payan D G, Brewster D R, Goetzl E J. J Immunol. 1983;131:1613–1615. [PubMed] [Google Scholar]
- 43.Stanisz A M, Befus D, Bienenstock J. J Immunol. 1986;136:152–156. [PubMed] [Google Scholar]