Abstract
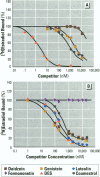
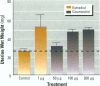
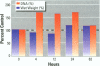
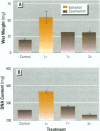
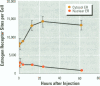
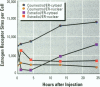
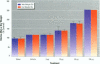
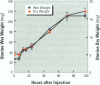
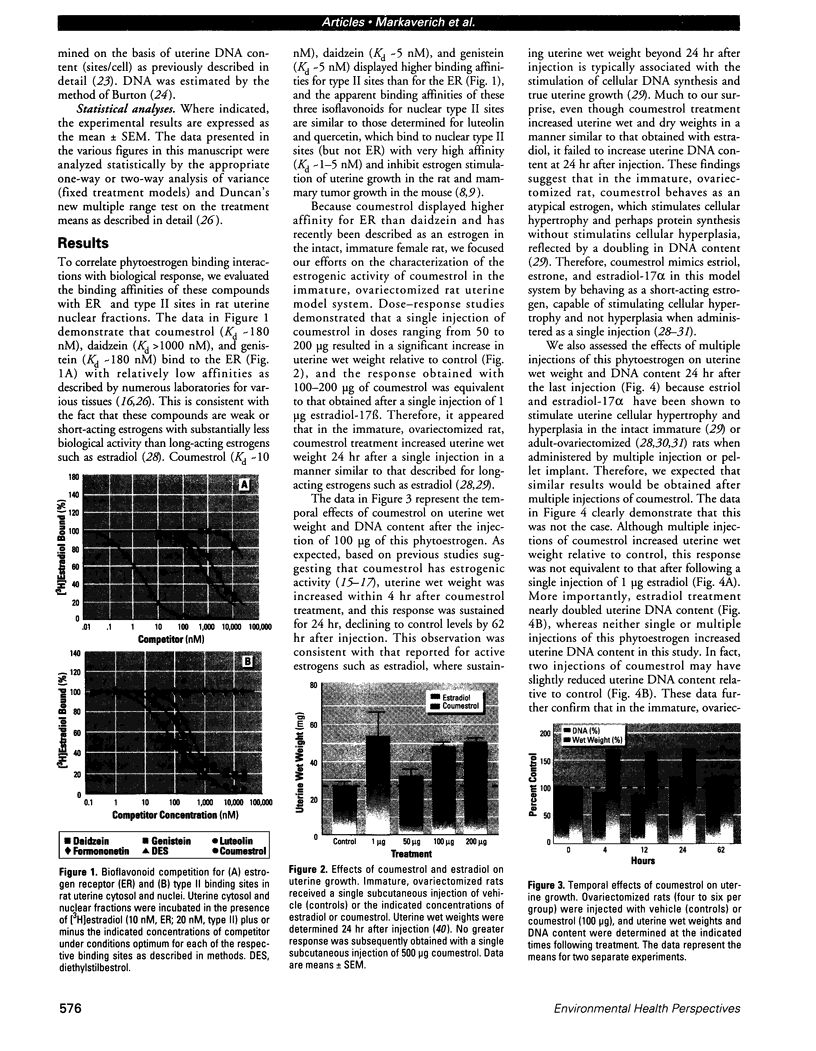
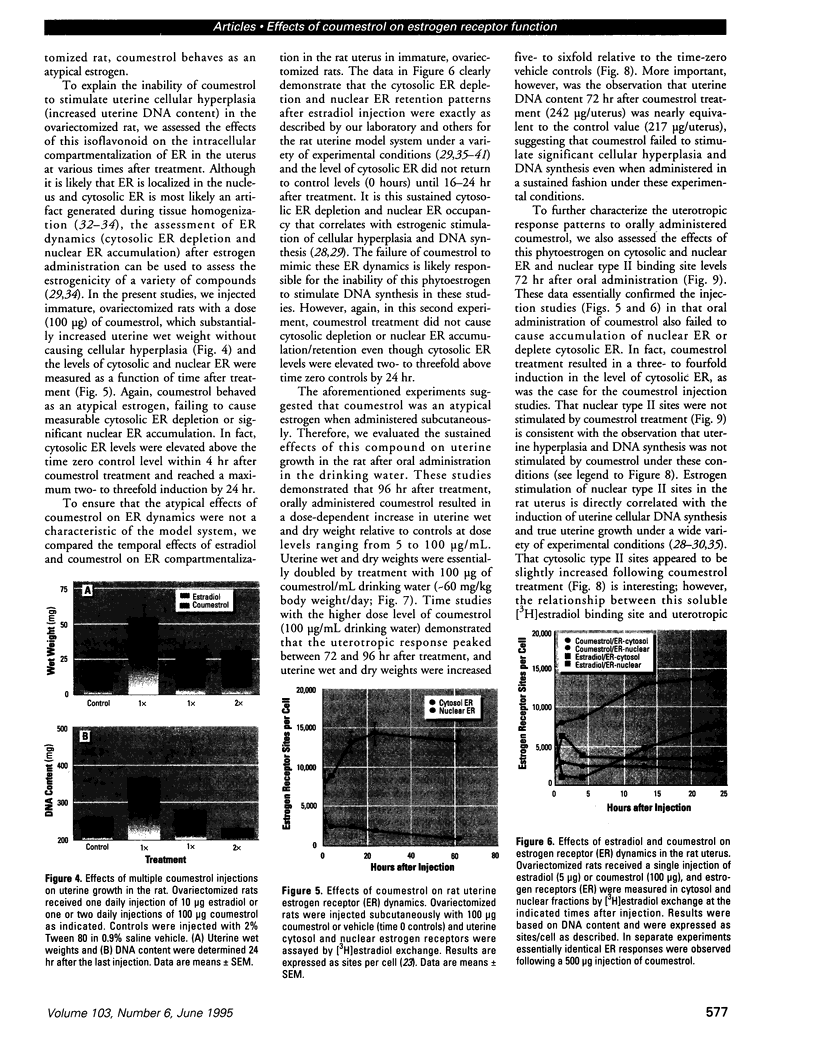
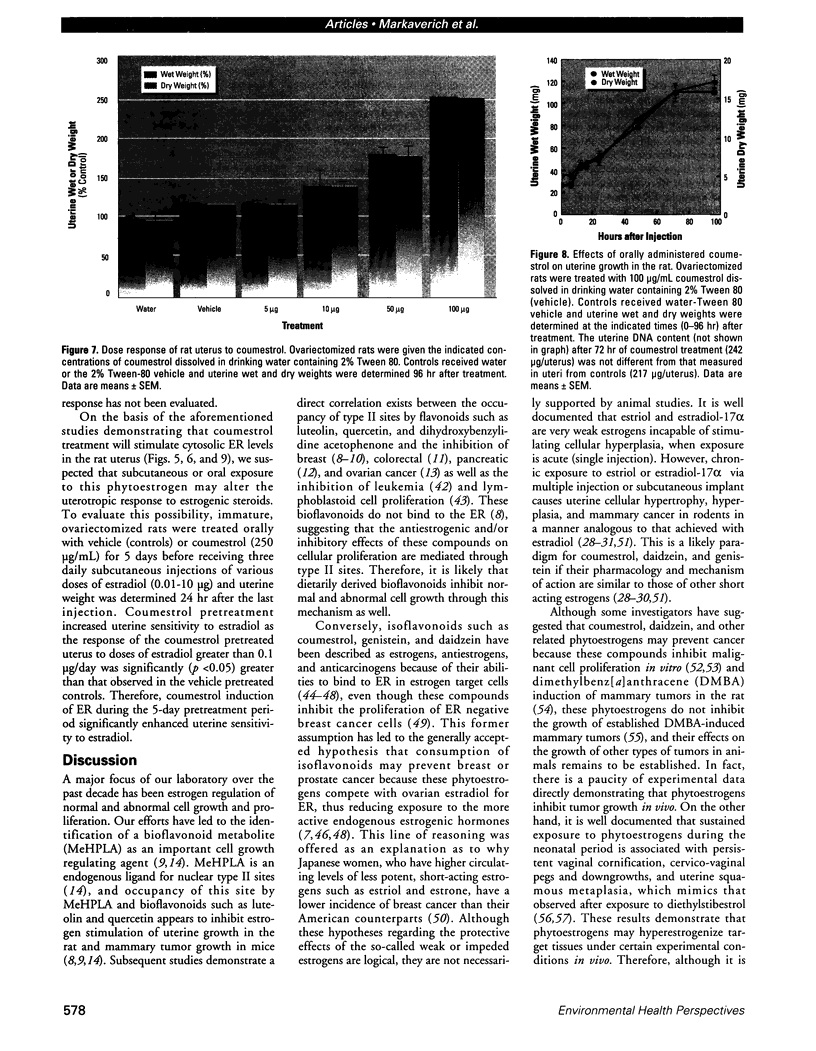
Isoflavonoids and related compounds such as coumestrol have classically been categorized as phytoestrogens because these environmentally derived substances bind to the estrogen receptor (ER) and increase uterine wet weight in immature rats and mice. Assessment of the binding affinities of isoflavonoids for ER and subsequent effects on uterine growth suggest these compounds are less active estrogens than estradiol and therefore may reduce the risk of developing breast or prostate cancer in humans by preventing estradiol binding to ER. With the renewed interest in the relationships between environmental estrogens and cancer cause and prevention, we assessed the effects of the phytoestrogen coumestrol on uterotropic response in the immature, ovariectomized rat. Our studies demonstrated that in this animal model, coumestrol is an atypical estrogen that does not stimulate uterine cellular hyperplasia. Although acute (subcutaneous injection) or chronic (multiple injection or orally via drinking water) administration of coumestrol significantly increased uterine wet and dry weights, the phytoestrogen failed to increase uterine DNA content. The lack of true estrogenic activity was characterized by the inability of this phytoestrogen to cause cytosolic ER depletion, nuclear ER accumulation, or the stimulation of nuclear type II sites which characteristically precede estrogenic stimulation of cellular DNA synthesis and proliferation. In fact, subcutaneous or oral coumestrol treatment caused an atypical threefold induction of cytosolic ER without corresponding cytosolic depletion and nuclear accumulation of this receptor, and this increased the sensitivity of the uterus to subsequent stimulation by estradiol.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H., Markkanen H., Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993 Nov 13;342(8881):1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Markkanen H., Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993 Nov 13;342(8881):1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Mousavi Y., Höckerstedt K. Diet and breast cancer. Acta Oncol. 1992;31(2):175–181. doi: 10.3109/02841869209088899. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Mousavi Y., Höckerstedt K. Diet and breast cancer. Acta Oncol. 1992;31(2):175–181. doi: 10.3109/02841869209088899. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs C. D., Bern H. A., Stokstad E. L. Prolonged vaginal cornification and other changes in mice treated neonatally with coumestrol, a plant estrogen. J Toxicol Environ Health. 1985;15(1):51–61. doi: 10.1080/15287398509530635. [DOI] [PubMed] [Google Scholar]
- Burroughs C. D., Mills K. T., Bern H. A. Reproductive abnormalities in female mice exposed neonatally to various doses of coumestrol. J Toxicol Environ Health. 1990 Jun;30(2):105–122. doi: 10.1080/15287399009531415. [DOI] [PubMed] [Google Scholar]
- Carbone A., Ranelletti F. O., Rinelli A., Vecchio F. M., Lauriola L., Piantelli M., Capelli A. Type II estrogen receptors in the papillary cystic tumor of the pancreas. Am J Clin Pathol. 1989 Nov;92(5):572–576. doi: 10.1093/ajcp/92.5.572. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Paszko Z., Peck E. J., Jr Nuclear binding and retention of the receptor estrogen complex: relation to the agonistic and antagonistic properties of estriol. Endocrinology. 1977 Jan;100(1):91–96. doi: 10.1210/endo-100-1-91. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Williams M., Upchurch S., Eriksson H., Helton E., Markaverich B. M. Effects of estradiol-17 alpha on nuclear occupancy of the estrogen receptor, stimulation of nuclear type II sites and uterine growth. J Steroid Biochem. 1982 Feb;16(2):323–328. doi: 10.1016/0022-4731(82)90184-4. [DOI] [PubMed] [Google Scholar]
- Ferguson E. R., Katzenellenbogen B. S. A comparative study of antiestrogen action: temporal patterns of antagonism of estrogen stimulated uterine growth and effects on estrogen receptor levels. Endocrinology. 1977 May;100(5):1242–1251. doi: 10.1210/endo-100-5-1242. [DOI] [PubMed] [Google Scholar]
- Fischer S. M., Cameron G. S., Baldwin J. K., Jasheway D. W., Patrick K. E. Reactive oxygen in the tumor promotion stage of skin carcinogenesis. Lipids. 1988 Jun;23(6):592–597. doi: 10.1007/BF02535603. [DOI] [PubMed] [Google Scholar]
- Graham S., Dayal H., Swanson M., Mittelman A., Wilkinson G. Diet in the epidemiology of cancer of the colon and rectum. J Natl Cancer Inst. 1978 Sep;61(3):709–714. [PubMed] [Google Scholar]
- Haenszel W., Locke F. B., Segi M. A case-control study of large bowel cancer in Japan. J Natl Cancer Inst. 1980 Jan;64(1):17–22. [PubMed] [Google Scholar]
- Jordan V. C., Tate A. C., Lyman S. D., Gosden B., Wolf M. F., Bain R. R., Welshons W. V. Rat uterine growth and induction of progesterone receptor without estrogen receptor translocation. Endocrinology. 1985 May;116(5):1845–1857. doi: 10.1210/endo-116-5-1845. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Ferguson E. R. Antiestrogen action in the uterus: biological ineffectiveness of nuclear bound estradiol after antiestrogen. Endocrinology. 1975 Jul;97(1):1–12. doi: 10.1210/endo-97-1-1. [DOI] [PubMed] [Google Scholar]
- Kaziro R., Kennedy J. P., Cole E. R., Southwell-Keely P. T. The oestrogenicity of equol in sheep. J Endocrinol. 1984 Dec;103(3):395–399. doi: 10.1677/joe.0.1030395. [DOI] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Koseki Y., Zava D. T., Chamness G. C., McGuire W. L. Estrogen receptor translocation and replenishment by the antiestrogen tamoxifen. Endocrinology. 1977 Oct;101(4):1104–1110. doi: 10.1210/endo-101-4-1104. [DOI] [PubMed] [Google Scholar]
- Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- Lamartiniere C. A., Moore J., Holland M., Barnes S. Neonatal genistein chemoprevents mammary cancer. Proc Soc Exp Biol Med. 1995 Jan;208(1):120–123. doi: 10.3181/00379727-208-43843. [DOI] [PubMed] [Google Scholar]
- Lan N. C., Katzenellenbogen B. S. Temporal relationships between hormone receptor binding and biological responses in the uterus: studies with short- and long-acting derivatives of estriol. Endocrinology. 1976 Jan;98(1):220–227. doi: 10.1210/endo-98-1-220. [DOI] [PubMed] [Google Scholar]
- Makela S, Davis VL, Tally WC, Korkman J, Salo L, Vihko R, Santti R, Korach KS. Dietary Estrogens Act through Estrogen Receptor-Mediated Processes and Show No Antiestrogenicity in Cultured Breast Cancer Cells. Environ Health Perspect. 1994 Jun;102(6-7):572–578. doi: 10.1289/ehp.94102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markaverich B. M., Adams N. R., Roberts R. R., Alejandro M., Clark J. H. Cytosol type II sites in the rat uterus: interaction with an endogenous ligand. J Steroid Biochem. 1987 Dec;28(6):599–608. doi: 10.1016/0022-4731(87)90386-4. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Clark J. H. Two binding sites for estradiol in rat uterine nuclei: relationship to uterotropic response. Endocrinology. 1979 Dec;105(6):1458–1462. doi: 10.1210/endo-105-6-1458. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Gregory R. R., Alejandro M. A., Clark J. H., Johnson G. A., Middleditch B. S. Methyl p-hydroxyphenyllactate. An inhibitor of cell growth and proliferation and an endogenous ligand for nuclear type-II binding sites. J Biol Chem. 1988 May 25;263(15):7203–7210. [PubMed] [Google Scholar]
- Markaverich B. M., Gregory R. R., Alejandro M., Kittrell F. S., Medina D., Clark J. H., Varma M., Varma R. S. Methyl p-hydroxyphenyllactate and nuclear type II binding sites in malignant cells: metabolic fate and mammary tumor growth. Cancer Res. 1990 Mar 1;50(5):1470–1478. [PubMed] [Google Scholar]
- Markaverich B. M., Roberts R. R., Alejandro M. A., Clark J. H. An endogenous inhibitor of [3H]estradiol binding to nuclear type II estrogen binding sites in normal and malignant tissues. Cancer Res. 1984 Apr;44(4):1515–1519. [PubMed] [Google Scholar]
- Markaverich B. M., Roberts R. R., Alejandro M. A., Johnson G. A., Middleditch B. S., Clark J. H. Bioflavonoid interaction with rat uterine type II binding sites and cell growth inhibition. J Steroid Biochem. 1988;30(1-6):71–78. doi: 10.1016/0022-4731(88)90078-7. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Roberts R. R., Alejandro M., Clark J. H. The effect of low dose continuous exposure to estradiol on the estrogen receptor (type I) and nuclear type II sites. Endocrinology. 1984 Mar;114(3):814–820. doi: 10.1210/endo-114-3-814. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Roberts R. R., Finney R. W., Clark J. H. Preliminary characterization of an endogenous inhibitor of [3H]estradiol binding in rat uterine nuclei. J Biol Chem. 1983 Oct 10;258(19):11663–11671. [PubMed] [Google Scholar]
- Markaverich B. M., Upchurch S., Clark J. H. Progesterone and dexamethasone antagonism of uterine growth: a role for a second nuclear binding site for estradiol in estrogen action. J Steroid Biochem. 1981 Feb;14(2):125–132. doi: 10.1016/0022-4731(81)90164-3. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Williams M., Upchurch S., Clark J. H. Heterogeneity of nuclear estrogen-binding sites in the rat uterus: a simple method for the quantitation of type I and type II sites by [3H]estradiol exchange. Endocrinology. 1981 Jul;109(1):62–69. doi: 10.1210/endo-109-1-62. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Pagano M., De Goeij C. C., Di Domenico M., Castoria G., Sluyser M., Auricchio F. Phosphorylation and estradiol binding of estrogen receptor in hormone-dependent and hormone-independent GR mouse mammary tumors. Int J Cancer. 1992 Jul 9;51(5):733–739. doi: 10.1002/ijc.2910510512. [DOI] [PubMed] [Google Scholar]
- Miksicek R. J. Commonly occurring plant flavonoids have estrogenic activity. Mol Pharmacol. 1993 Jul;44(1):37–43. [PubMed] [Google Scholar]
- Monti E., Sinha B. K. Antiproliferative effect of genistein and adriamycin against estrogen-dependent and -independent human breast carcinoma cell lines. Anticancer Res. 1994 May-Jun;14(3A):1221–1226. [PubMed] [Google Scholar]
- Noble R. L., Hochachka B. C., King D. Spontaneous and estrogen-produced tumors in Nb rats and their behavior after transplantation. Cancer Res. 1975 Mar;35(3):766–780. [PubMed] [Google Scholar]
- Ortí E., Bodwell J. E., Munck A. Phosphorylation of steroid hormone receptors. Endocr Rev. 1992 Feb;13(1):105–128. doi: 10.1210/edrv-13-1-105. [DOI] [PubMed] [Google Scholar]
- Pagliacci M. C., Spinozzi F., Migliorati G., Fumi G., Smacchia M., Grignani F., Riccardi C., Nicoletti I. Genistein inhibits tumour cell growth in vitro but enhances mitochondrial reduction of tetrazolium salts: a further pitfall in the use of the MTT assay for evaluating cell growth and survival. Eur J Cancer. 1993;29A(11):1573–1577. doi: 10.1016/0959-8049(93)90297-s. [DOI] [PubMed] [Google Scholar]
- Peterson G., Barnes S. Genistein inhibition of the growth of human breast cancer cells: independence from estrogen receptors and the multi-drug resistance gene. Biochem Biophys Res Commun. 1991 Aug 30;179(1):661–667. doi: 10.1016/0006-291x(91)91423-a. [DOI] [PubMed] [Google Scholar]
- Piantelli M., Ricci R., Larocca L. M., Rinelli A., Capelli A., Rizzo S., Scambia G., Ranelletti F. O. Type II oestrogen binding sites in human colorectal carcinoma. J Clin Pathol. 1990 Dec;43(12):1004–1006. doi: 10.1136/jcp.43.12.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruh T. S., Baudendistel L. J. Antiestrogen modulation of the salt-resistant nuclear estrogen receptor. Endocrinology. 1978 Jun;102(6):1838–1846. doi: 10.1210/endo-102-6-1838. [DOI] [PubMed] [Google Scholar]
- Ruh T. S., Baudendistel L. J. Different nuclear binding sites for antiestrogen and estrogen receptor complexes. Endocrinology. 1977 Feb;100(2):420–426. doi: 10.1210/endo-100-2-420. [DOI] [PubMed] [Google Scholar]
- Scambia G., Ranelletti F. O., Benedetti Panici P., Piantelli M., Bonanno G., De Vincenzo R., Ferrandina G., Pierelli L., Capelli A., Mancuso S. Quercetin inhibits the growth of a multidrug-resistant estrogen-receptor-negative MCF-7 human breast-cancer cell line expressing type II estrogen-binding sites. Cancer Chemother Pharmacol. 1991;28(4):255–258. doi: 10.1007/BF00685531. [DOI] [PubMed] [Google Scholar]
- Scambia G., Ranelletti F. O., Benedetti Panici P., Piantelli M., Rumi C., Battaglia F., Larocca L. M., Capelli A., Mancuso S. Type-II estrogen binding sites in a lymphoblastoid cell line and growth-inhibitory effect of estrogen, anti-estrogen and bioflavonoids. Int J Cancer. 1990 Dec 15;46(6):1112–1116. doi: 10.1002/ijc.2910460627. [DOI] [PubMed] [Google Scholar]
- Scambia G., Ranelletti F. O., Panici P. B., Piantelli M., Bonanno G., De Vincenzo R., Ferrandina G., Rumi C., Larocca L. M., Mancuso S. Inhibitory effect of quercetin on OVCA 433 cells and presence of type II oestrogen binding sites in primary ovarian tumours and cultured cells. Br J Cancer. 1990 Dec;62(6):942–946. doi: 10.1038/bjc.1990.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma O. P., Adlercreutz H., Strandberg J. D., Zirkin B. R., Coffey D. S., Ewing L. L. Soy of dietary source plays a preventive role against the pathogenesis of prostatitis in rats. J Steroid Biochem Mol Biol. 1992 Nov;43(6):557–564. doi: 10.1016/0960-0760(92)90244-d. [DOI] [PubMed] [Google Scholar]
- Shutt D. A. The effects of plant oestrogens on animal reproduction. Endeavour. 1976 Sep;35(126):110–113. doi: 10.1016/0160-9327(76)90004-1. [DOI] [PubMed] [Google Scholar]
- Tang B. Y., Adams N. R. Effect of equol on oestrogen receptors and on synthesis of DNA and protein in the immature rat uterus. J Endocrinol. 1980 May;85(2):291–297. doi: 10.1677/joe.0.0850291. [DOI] [PubMed] [Google Scholar]
- Teofili L., Pierelli L., Iovino M. S., Leone G., Scambia G., De Vincenzo R., Benedetti-Panici P., Menichella G., Macrì E., Piantelli M. The combination of quercetin and cytosine arabinoside synergistically inhibits leukemic cell growth. Leuk Res. 1992;16(5):497–503. doi: 10.1016/0145-2126(92)90176-8. [DOI] [PubMed] [Google Scholar]
- Verdeal K., Brown R. R., Richardson T., Ryan D. S. Affinity of phytoestrogens for estradiol-binding proteins and effect of coumestrol on growth of 7,12-dimethylbenz[a]anthracene-induced rat mammary tumors. J Natl Cancer Inst. 1980 Feb;64(2):285–290. doi: 10.1093/jnci/64.2.285. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- Whitten P. L., Naftolin F. Effects of a phytoestrogen diet on estrogen-dependent reproductive processes in immature female rats. Steroids. 1992 Feb;57(2):56–61. doi: 10.1016/0039-128x(92)90029-9. [DOI] [PubMed] [Google Scholar]
- Whitten P. L., Russell E., Naftolin F. Effects of a normal, human-concentration, phytoestrogen diet on rat uterine growth. Steroids. 1992 Mar;57(3):98–106. doi: 10.1016/0039-128x(92)90066-i. [DOI] [PubMed] [Google Scholar]
- Whitten P. L., Russell E., Naftolin F. Influence of phytoestrogen diets on estradiol action in the rat uterus. Steroids. 1994 Jul;59(7):443–449. doi: 10.1016/0039-128x(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Xu X., Wang H. J., Murphy P. A., Cook L., Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994 Jun;124(6):825–832. doi: 10.1093/jn/124.6.825. [DOI] [PubMed] [Google Scholar]