Abstract
Cancer is a worldwide public health concern. Identifying carcinogens and limiting their exposure is one approach to the problem of reducing risk. Currently, epidemiology and rodent bioassays are the means by which putative human carcinogens are identified. Both methods have intrinsic limitations: they are slow and expensive processes with many uncertainties. The development of methods to modify specific genes in the mammalian genome has provided promising new tools for identifying carcinogens and characterizing risk. Transgenic mice may provide advantages in shortening the time required for bioassays and improving the accuracy of carcinogen identification; transgenic mice might now be included in the testing armamentarium without abandoning the two-year bioassay, the current standard. We show that mutagenic carcinogens can be identified with increased sensitivity and specificity using hemizygous p53 mice in which one allele of the p53 gene has been inactivated. Furthermore, the TG.AC transgenic model, carrying a v-Ha-ras construct, has developed papillomas and malignant tumors in response to a number of mutagenic and nonmutagenic carcinogens and tumor promoters, but not to noncarcinogens. We present a decision-tree approach that permits, at modest extra cost, the testing of more chemicals with improved ability to extrapolate from rodents to humans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Tennant R. W. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat Res. 1988 Jan;204(1):17–115. doi: 10.1016/0165-1218(88)90114-0. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Definitive relationships among chemical structure, carcinogenicity and mutagenicity for 301 chemicals tested by the U.S. NTP. Mutat Res. 1991 May;257(3):229–306. doi: 10.1016/0165-1110(91)90003-e. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Prediction of rodent carcinogenicity for 44 chemicals: results. Mutagenesis. 1994 Jan;9(1):7–15. doi: 10.1093/mutage/9.1.7. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W., Zeiger E., Stasiewicz S. Classification according to chemical structure, mutagenicity to Salmonella and level of carcinogenicity of a further 42 chemicals tested for carcinogenicity by the U.S. National Toxicology Program. Mutat Res. 1989 Jun;223(2):73–103. doi: 10.1016/0165-1218(89)90037-2. [DOI] [PubMed] [Google Scholar]
- BOUTWELL R. K. SOME BIOLOGICAL ASPECTS OF SKIN CARCINOGENISIS. Prog Exp Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
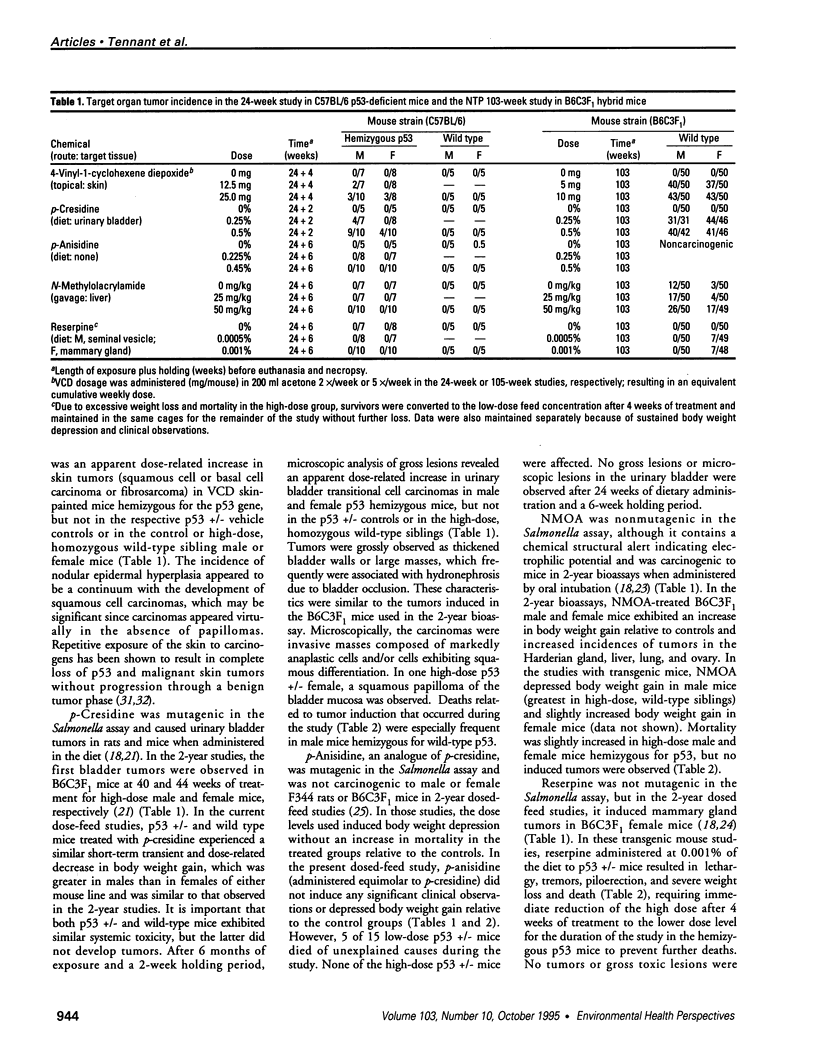
- Chhabra R. S., Huff J., Haseman J., Jokinen M. P., Hetjmancik M. Dermal toxicity and carcinogenicity of 4-vinyl-1-cyclohexene diepoxide in Fischer rats and B6C3F1 mice. Fundam Appl Toxicol. 1990 May;14(4):752–763. doi: 10.1016/0272-0590(90)90300-9. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Frederick C. B., Potter D. W., Chang-Mateu M. I., Andersen M. E. A physiologically based pharmacokinetic and pharmacodynamic model to describe the oral dosing of rats with ethyl acrylate and its implications for risk assessment. Toxicol Appl Pharmacol. 1992 Jun;114(2):246–260. doi: 10.1016/0041-008x(92)90075-4. [DOI] [PubMed] [Google Scholar]
- Ghanayem B. I., Maronpot R. R., Matthews H. B. Ethyl acrylate-induced gastric toxicity. III. Development and recovery of lesions. Toxicol Appl Pharmacol. 1986 May;83(3):576–583. doi: 10.1016/0041-008x(86)90240-1. [DOI] [PubMed] [Google Scholar]
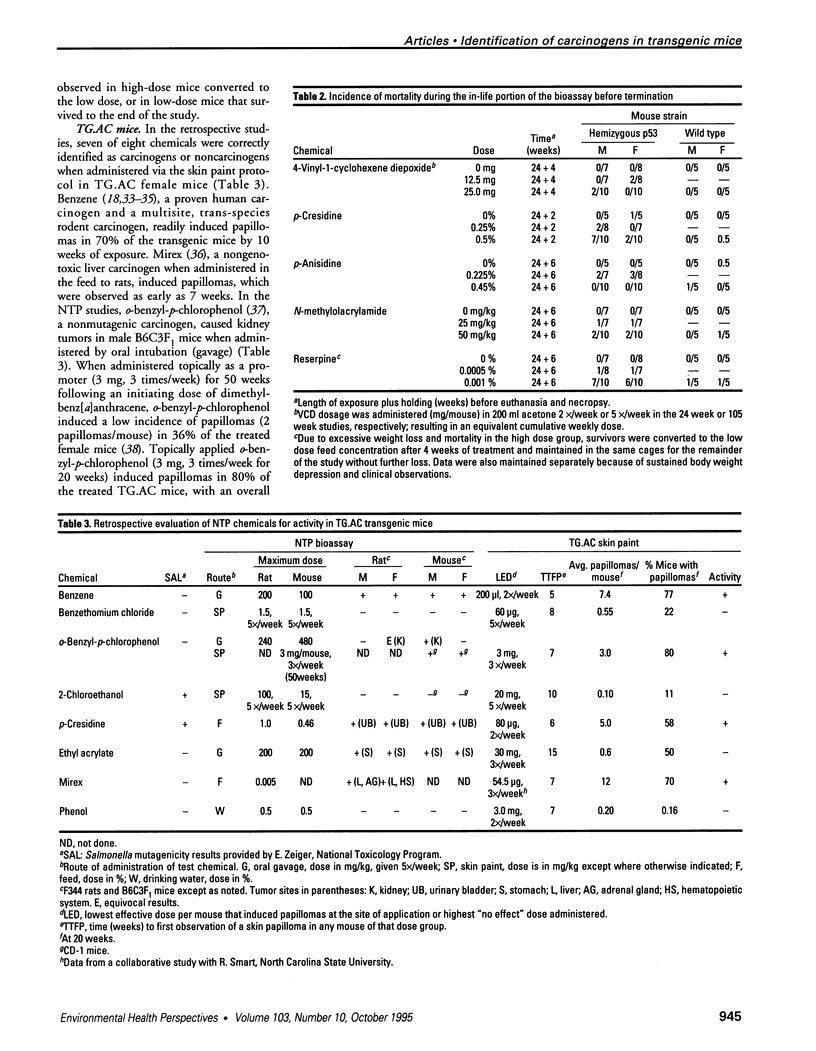
- Hansen L. A., Tennant R. Focal transgene expression associated with papilloma development in v-Ha-ras-transgenic TG.AC mice. Mol Carcinog. 1994 Mar;9(3):143–154. doi: 10.1002/mc.2940090306. [DOI] [PubMed] [Google Scholar]
- Harris C. C. p53: at the crossroads of molecular carcinogenesis and risk assessment. Science. 1993 Dec 24;262(5142):1980–1981. doi: 10.1126/science.8266092. [DOI] [PubMed] [Google Scholar]
- Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992 Nov 13;71(4):543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- Harvey M., McArthur M. J., Montgomery C. A., Jr, Bradley A., Donehower L. A. Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J. 1993 Jul;7(10):938–943. doi: 10.1096/fasebj.7.10.8344491. [DOI] [PubMed] [Google Scholar]
- Harvey M., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A., Donehower L. A. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet. 1993 Nov;5(3):225–229. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J. E., Rao G. N., Arnold J. E., Boorman G. A., McConnell E. E. Neoplasms observed in untreated and corn oil gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst. 1985 Nov;75(5):975–984. doi: 10.1093/jnci/75.5.975. [DOI] [PubMed] [Google Scholar]
- Hegi M. E., Söderkvist P., Foley J. F., Schoonhoven R., Swenberg J. A., Kari F., Maronpot R., Anderson M. W., Wiseman R. W. Characterization of p53 mutations in methylene chloride-induced lung tumors from B6C3F1 mice. Carcinogenesis. 1993 May;14(5):803–810. doi: 10.1093/carcin/14.5.803. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Huff J. E., Haseman J. K., DeMarini D. M., Eustis S., Maronpot R. R., Peters A. C., Persing R. L., Chrisp C. E., Jacobs A. C. Multiple-site carcinogenicity of benzene in Fischer 344 rats and B6C3F1 mice. Environ Health Perspect. 1989 Jul;82:125–163. doi: 10.1289/ehp.8982125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J., Haseman J., Rall D. Scientific concepts, value, and significance of chemical carcinogenesis studies. Annu Rev Pharmacol Toxicol. 1991;31:621–652. doi: 10.1146/annurev.pa.31.040191.003201. [DOI] [PubMed] [Google Scholar]
- Jerry D. J., Butel J. S., Donehower L. A., Paulson E. J., Cochran C., Wiseman R. W., Medina D. Infrequent p53 mutations in 7,12-dimethylbenz[a]anthracene-induced mammary tumors in BALB/c and p53 hemizygous mice. Mol Carcinog. 1994 Mar;9(3):175–183. doi: 10.1002/mc.2940090309. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kemp C. J., Donehower L. A., Bradley A., Balmain A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell. 1993 Sep 10;74(5):813–822. doi: 10.1016/0092-8674(93)90461-x. [DOI] [PubMed] [Google Scholar]
- Lave L. B., Ennever F. K., Rosenkranz H. S., Omenn G. S. Information value of the rodent bioassay. Nature. 1988 Dec 15;336(6200):631–633. doi: 10.1038/336631a0. [DOI] [PubMed] [Google Scholar]
- Lave L. B., Omenn G. S. Cost-effectiveness of short-term tests for carcinogenicity. Nature. 1986 Nov 6;324(6092):29–34. doi: 10.1038/324029a0. [DOI] [PubMed] [Google Scholar]
- Leder A., Kuo A., Cardiff R. D., Sinn E., Leder P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9178–9182. doi: 10.1073/pnas.87.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Mirsalis J. C., Monforte J. A., Winegar R. A. Transgenic animal models for measuring mutations in vivo. Crit Rev Toxicol. 1994;24(3):255–280. doi: 10.3109/10408449409021608. [DOI] [PubMed] [Google Scholar]
- Morrison V., Ashby J. A preliminary evaluation of the performance of the Muta Mouse (lacZ) and Big Blue (lacI) transgenic mouse mutation assays. Mutagenesis. 1994 Jul;9(4):367–375. doi: 10.1093/mutage/9.4.367. [DOI] [PubMed] [Google Scholar]
- Omenn G. S., Lave L. B. Scientific and cost-effectiveness criteria in selecting batteries of short-term tests. Mutat Res. 1988 May-Aug;205(1-4):41–49. doi: 10.1016/0165-1218(88)90007-9. [DOI] [PubMed] [Google Scholar]
- Shelby M. D., Zeiger E. Activity of human carcinogens in the Salmonella and rodent bone-marrow cytogenetics tests. Mutat Res. 1990 Jun-Aug;234(3-4):257–261. doi: 10.1016/0165-1161(90)90022-g. [DOI] [PubMed] [Google Scholar]
- Spalding J. W., Momma J., Elwell M. R., Tennant R. W. Chemically induced skin carcinogenesis in a transgenic mouse line (TG.AC) carrying a v-Ha-ras gene. Carcinogenesis. 1993 Jul;14(7):1335–1341. doi: 10.1093/carcin/14.7.1335. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Stoler A. B., Stenback F., Balmain A. The conversion of mouse skin squamous cell carcinomas to spindle cell carcinomas is a recessive event. J Cell Biol. 1993 Sep;122(5):1103–1117. doi: 10.1083/jcb.122.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Ashby J. Classification according to chemical structure, mutagenicity to Salmonella and level of carcinogenicity of a further 39 chemicals tested for carcinogenicity by the U.S. National Toxicology Program. Mutat Res. 1991 May;257(3):209–227. doi: 10.1016/0165-1110(91)90002-d. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Margolin B. H., Shelby M. D., Zeiger E., Haseman J. K., Spalding J., Caspary W., Resnick M., Stasiewicz S., Anderson B. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–941. doi: 10.1126/science.3554512. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Rao G. N., Russfield A., Seilkop S., Braun A. G. Chemical effects in transgenic mice bearing oncogenes expressed in mammary tissue. Carcinogenesis. 1993 Jan;14(1):29–35. doi: 10.1093/carcin/14.1.29. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Spalding J., Stasiewicz S., Ashby J. Prediction of the outcome of rodent carcinogenicity bioassays currently being conducted on 44 chemicals by the National Toxicology Program. Mutagenesis. 1990 Jan;5(1):3–14. doi: 10.1093/mutage/5.1.3. [DOI] [PubMed] [Google Scholar]
- Tennant R. W. Stratification of rodent carcinogenicity bioassay results to reflect relative human hazard. Mutat Res. 1993 Mar;286(1):111–118. doi: 10.1016/0027-5107(93)90006-2. [DOI] [PubMed] [Google Scholar]
- Van Duuren B. L., Orris L., Nelson N. Carcinogenicity of epoxides, lactones, and peroxy compounds. II. J Natl Cancer Inst. 1965 Oct;35(4):707–717. [PubMed] [Google Scholar]
- Vogelstein B. Cancer. A deadly inheritance. Nature. 1990 Dec 20;348(6303):681–682. doi: 10.1038/348681a0. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Zambetti G. P., Levine A. J. A comparison of the biological activities of wild-type and mutant p53. FASEB J. 1993 Jul;7(10):855–865. doi: 10.1096/fasebj.7.10.8344485. [DOI] [PubMed] [Google Scholar]
- Zeiger E., Haseman J. K., Shelby M. D., Margolin B. H., Tennant R. W. Evaluation of four in vitro genetic toxicity tests for predicting rodent carcinogenicity: confirmation of earlier results with 41 additional chemicals. Environ Mol Mutagen. 1990;16 (Suppl 18):1–14. doi: 10.1002/em.2850160502. [DOI] [PubMed] [Google Scholar]