Abstract
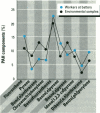
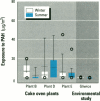
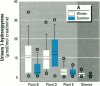
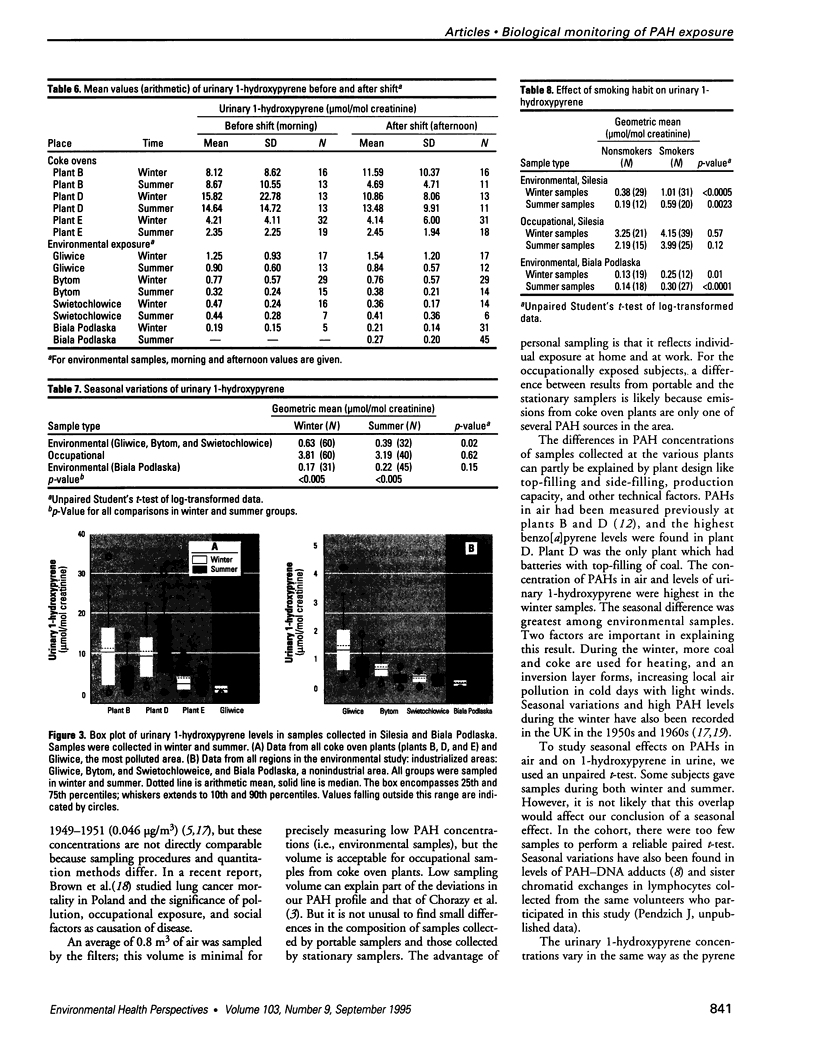
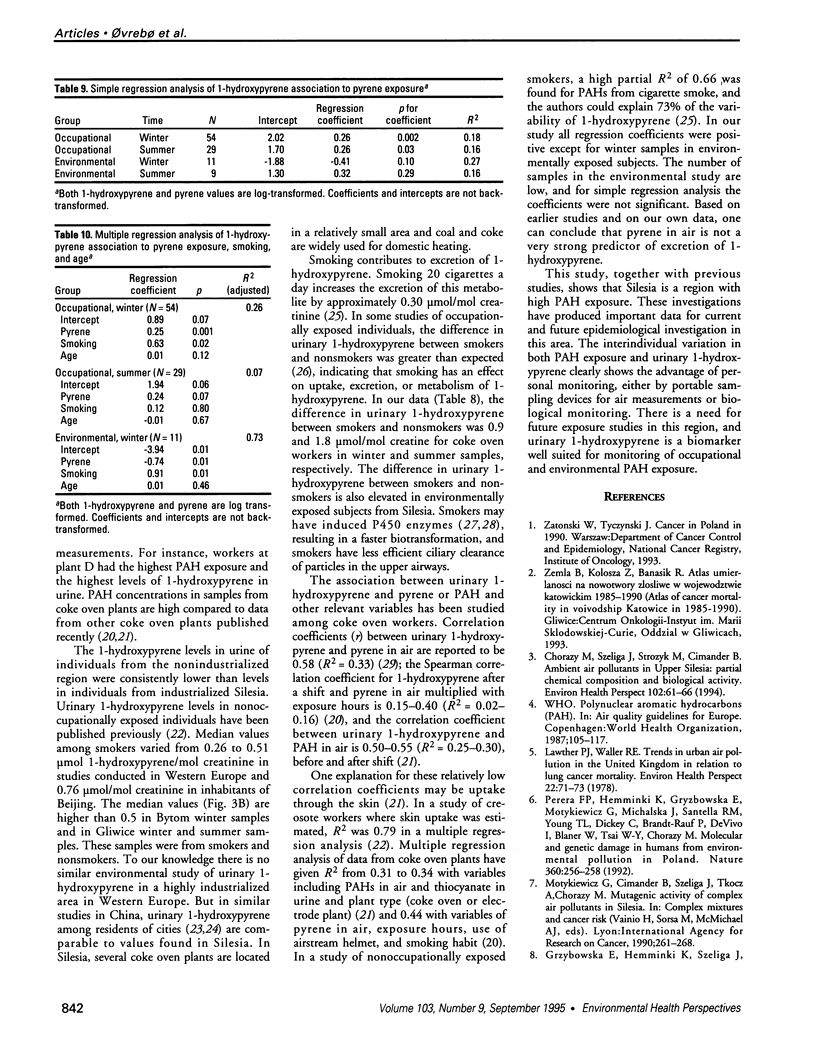
Air pollution in Poland and particularly in Silesia is among the worst in Europe. Many coal mines and coke oven plants are located in this area, representing a major source of carcinogenic polycyclic aromatic hydrocarbons (PAHs). We quantitated the PAH exposure level in air samples using personal sampling devices, collected urine samples from the same individuals, and measured 1-hydroxypyrene with high performance liquid chromatography. Samples were collected twice, once in February and once in September. Mean PAH level of samples collected at three different coke oven plants varied from 2.3 micrograms/m3 to 12.3 micrograms/m3; the lowest mean was in September. Mean levels of 0.15 micrograms/m3 (September) and 0.44 micrograms/m3 (February) were noted for the environmentally exposed group. Mean urinary 1-hydroxypyrene varied from 2.45 to 13.48 mumol/mol creatinine at the three coke oven plants. The corresponding variation between the three different environmentally exposed groups in Silesia was 0.41-1.54 mumol/mol creatinine. In the nonindustrialized area, the mean varied from 0.20 to 0.14 mumol/mol creatinine. Seasonal variation was found both at the coke oven plants and in the environmental exposed groups in Silesia. Both PAH levels and 1-hydroxypyrene varied seasonally among coke oven workers and the environmentally exposed group. Our study shows that PAH exposure in the industrialized area of Silesia is high compared to levels in Western Europe. 1-Hydroxypyrene excretion in environmentally exposed individuals in Poland is among the highest in Europe.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessio L., Berlin A., Dell'Orto A., Toffoletto F., Ghezzi I. Reliability of urinary creatinine as a parameter used to adjust values of urinary biological indicators. Int Arch Occup Environ Health. 1985;55(2):99–106. doi: 10.1007/BF00378371. [DOI] [PubMed] [Google Scholar]
- Brown H. S., Goble R., Kirschner H. Social and environmental factors in lung cancer mortality in post-war Poland. Environ Health Perspect. 1995 Jan;103(1):64–70. doi: 10.1289/ehp.9510364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorazy M., Szeliga J., Strózyk M., Cimander B. Ambient air pollutants in upper Silesia: partial chemical composition and biological activity. Environ Health Perspect. 1994 Oct;102 (Suppl 4):61–66. doi: 10.1289/ehp.94102s461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M., Jr, Buchet J. P., Burrion J. B., Moro J., Cupers L., Delavignette J. P., Jacques J., Lauwerys R. Determinants of urinary thioethers, D-glucaric acid and mutagenicity after exposure to polycyclic aromatic hydrocarbons assessed by air monitoring and measurement of 1-hydroxypyrene in urine: a cross-sectional study in workers of coke and graphite-electrode-producing plants. Int Arch Occup Environ Health. 1994;65(5):329–338. doi: 10.1007/BF00405698. [DOI] [PubMed] [Google Scholar]
- Grzybowska E., Hemminki K., Choraźy M. Seasonal variations in levels of DNA adducts and X-spots in human populations living in different parts of Poland. Environ Health Perspect. 1993 Mar;99:77–81. doi: 10.1289/ehp.939977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzybowska E., Hemminki K., Szeliga J., Chorazy M. Seasonal variation of aromatic DNA adducts in human lymphocytes and granulocytes. Carcinogenesis. 1993 Dec;14(12):2523–2526. doi: 10.1093/carcin/14.12.2523. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Henderson P. T. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr. 1987 Jan 23;413:227–232. doi: 10.1016/0378-4347(87)80230-x. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J. Biological exposure limit for occupational exposure to coal tar pitch volatiles at cokeovens. Int Arch Occup Environ Health. 1992;63(8):511–516. doi: 10.1007/BF00386338. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., van Leeuwen F. E., Oosterink S., Anzion R. B., van der Loop F., Bos R. P., van Veen H. G. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990 Jul;47(7):454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther P. J., Waller R. E. Trends in urban air pollution in the United Kingdom in relation to lung cancer mortality. Environ Health Perspect. 1978 Feb;22:71–73. doi: 10.1289/ehp.782271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovrebø S., Haugen A., Fjeldstad P. E., Hemminki K., Szyfter K. Biological monitoring of exposure to polycyclic aromatic hydrocarbon in an electrode paste plant. J Occup Med. 1994 Mar;36(3):303–310. doi: 10.1097/00043764-199403000-00007. [DOI] [PubMed] [Google Scholar]
- Perera F. P., Hemminki K., Gryzbowska E., Motykiewicz G., Michalska J., Santella R. M., Young T. L., Dickey C., Brandt-Rauf P., De Vivo I. Molecular and genetic damage in humans from environmental pollution in Poland. Nature. 1992 Nov 19;360(6401):256–258. doi: 10.1038/360256a0. [DOI] [PubMed] [Google Scholar]
- STOCKS P., COMMINS B. T., AUBREY K. V. A study of polycyclic hydrocarbons and trace elements in smoke in Merseyside and other northern localities. Int J Air Water Pollut. 1961 Sep;4:141–153. [PubMed] [Google Scholar]
- Van Rooij J. G., Van Lieshout E. M., Bodelier-Bade M. M., Jongeneelen F. J. Effect of the reduction of skin contamination on the internal dose of creosote workers exposed to polycyclic aromatic hydrocarbons. Scand J Work Environ Health. 1993 Jun;19(3):200–207. doi: 10.5271/sjweh.1322. [DOI] [PubMed] [Google Scholar]
- Van Rooij J. G., Veeger M. M., Bodelier-Bade M. M., Scheepers P. T., Jongeneelen F. J. Smoking and dietary intake of polycyclic aromatic hydrocarbons as sources of interindividual variability in the baseline excretion of 1-hydroxypyrene in urine. Int Arch Occup Environ Health. 1994;66(1):55–65. doi: 10.1007/BF00386580. [DOI] [PubMed] [Google Scholar]
- Vähäkangas K., Pyy L., Yrjänheikki E. Assessment of PAH-exposure among coke oven workers. Pharmacogenetics. 1992 Dec;2(6):304–308. [PubMed] [Google Scholar]
- WALLER R. E. The benzpyrene content of town air. Br J Cancer. 1952 Mar;6(1):8–21. doi: 10.1038/bjc.1952.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. H., Quan W. Y., Tian D. H. Experiments on the effects of several factors on the 1-hydroxypyrene level in human urine as an indicator of exposure to polycyclic aromatic hydrocarbons. Sci Total Environ. 1992 Mar 31;113(3):197–207. doi: 10.1016/0048-9697(92)90001-9. [DOI] [PubMed] [Google Scholar]
- Zhao Z. H., Quan W. Y., Tian D. H. Urinary 1-hydroxypyrene as an indicator of human exposure to ambient polycyclic aromatic hydrocarbons in a coal-burning environment. Sci Total Environ. 1990 Mar;92:145–154. doi: 10.1016/0048-9697(90)90326-p. [DOI] [PubMed] [Google Scholar]