Abstract
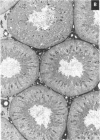
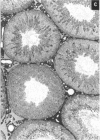
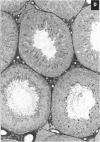
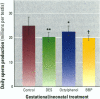
This study assessed whether exposure of male rats to two estrogenic, environmental chemicals, 4-octylphenol (OP) and butyl benzyl phthalate (BBP) during gestation or during the first 21 days of postnatal life, affected testicular size or spermatogenesis in adulthood (90-95 days of age). Chemicals were administered via the drinking water or concentrations of 10-1000 micrograms/l (OP) or 1000 micrograms/l (BBP), diethylstilbestrol (DES; 100 micrograms/l) and an octylphenol polyethoxylate (OPP; 1000 micrograms/l), which is a weak estrogen or nonestrogenic in vitro, were administered as presumptive positive and negative controls, respectively. Controls received the vehicle (ethanol) in tap water. In study 1, rats were treated from days 1-22 after births in studies 2 and 3, the mothers were treated for approximately 8-9 weeks, spanning a 2-week period before mating throughout gestation and 22 days after giving birth. With the exception of DES, treatment generally had no major adverse effect or body weight: in most instances, treated animals were heavier than controls at day 22 and at days 90-95. Exposure to OP, OPP, or BBP at a concentration of 1000 micrograms/1 resulted in a small (5-13%) but significant (p < 0.01 or p < 0.0001) reduction in mean testicular size in studies 2 and 3, an effect that was still evident when testicular weight was expressed relative to body, weight or kidney weight. The effect of OPP is attributed to its metabolism in vivo to OP. DES exposure caused similar reductions in testicular size but also caused reductions in body weight, kidney weight, and litter size. Ventral prostate weight was reduced significantly in DES-treated rats and to minor extent in OP-treated rats. Comparable but more minor effects of treatment with DES or OP on testicular size were observed in study 1. None of the treatments had any adverse effect on testicular morphology or on the cross-sectional area of the lumen or seminiferous epithelium at stages VII-VIII of the spermatogenic cycle, but DES, OP, and BBP caused reductions of 10-21% (p < 0.05 to p < 0.001) in daily sperm production. Humans are exposed to phthalates, such as BBP, and to alkylphenol polyethoxylates, such as OP, but to what extent is unknown. More detailed studies are warranted to assess the possible risk to the development of the human testis from exposure to these and other environmental estrogens.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal D. K., Maronpot R. R., Lamb J. C., 4th, Kluwe W. M. Adverse effects of butyl benzyl phthalate on the reproductive and hematopoietic systems of male rats. Toxicology. 1985 Jun 14;35(3):189–206. doi: 10.1016/0300-483x(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Auger J., Kunstmann J. M., Czyglik F., Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995 Feb 2;332(5):281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- Autian J. Toxicity and health threats of phthalate esters: review of the literature. Environ Health Perspect. 1973 Jun;4:3–26. doi: 10.1289/ehp.73043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromwich P., Cohen J., Stewart I., Walker A. Decline in sperm counts: an artefact of changed reference range of "normal"? BMJ. 1994 Jul 2;309(6946):19–22. doi: 10.1136/bmj.309.6946.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. S., Porcelli J., Hess R. A. Induction of increased testis growth and sperm production in adult rats by neonatal administration of the goitrogen propylthiouracil (PTU): the critical period. Biol Reprod. 1992 Jan;46(1):146–154. doi: 10.1095/biolreprod46.1.146. [DOI] [PubMed] [Google Scholar]
- Dostal L. A., Chapin R. E., Stefanski S. A., Harris M. W., Schwetz B. A. Testicular toxicity and reduced Sertoli cell numbers in neonatal rats by di(2-ethylhexyl)phthalate and the recovery of fertility as adults. Toxicol Appl Pharmacol. 1988 Aug;95(1):104–121. doi: 10.1016/s0041-008x(88)80012-7. [DOI] [PubMed] [Google Scholar]
- Dostal L. A., Weaver R. P., Schwetz B. A. Transfer of di(2-ethylhexyl) phthalate through rat milk and effects on milk composition and the mammary gland. Toxicol Appl Pharmacol. 1987 Dec;91(3):315–325. doi: 10.1016/0041-008x(87)90054-8. [DOI] [PubMed] [Google Scholar]
- Irvine D. S. Falling sperm quality. BMJ. 1994 Aug 13;309(6952):476–476. doi: 10.1136/bmj.309.6952.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L., Petty C. S., Neaves W. B. A comparative study of daily sperm production and testicular composition in humans and rats. Biol Reprod. 1980 Jun;22(5):1233–1243. doi: 10.1093/biolreprod/22.5.1233. [DOI] [PubMed] [Google Scholar]
- Johnson L., Petty C. S., Neaves W. B. A new approach to quantification of spermatogenesis and its application to germinal cell attrition during human spermiogenesis. Biol Reprod. 1981 Aug;25(1):217–226. doi: 10.1095/biolreprod25.1.217. [DOI] [PubMed] [Google Scholar]
- Kerr J. B., Sharpe R. M. Follicle-stimulating hormone induction of Leydig cell maturation. Endocrinology. 1985 Jun;116(6):2592–2604. doi: 10.1210/endo-116-6-2592. [DOI] [PubMed] [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993 Jun;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., CLERMONT Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952 Nov 20;55(4):548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Mayer F. L., Stalling D. L., Johnson J. L. Phthalate esters as environmental contaminants. Nature. 1972 Aug 18;238(5364):411–413. doi: 10.1038/238411a0. [DOI] [PubMed] [Google Scholar]
- Orth J. M., Gunsalus G. L., Lamperti A. A. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988 Mar;122(3):787–794. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- Page B. D., Lacroix G. M. Studies into the transfer and migration of phthalate esters from aluminium foil-paper laminates to butter and margarine. Food Addit Contam. 1992 May-Jun;9(3):197–212. doi: 10.1080/02652039209374064. [DOI] [PubMed] [Google Scholar]
- Sharman M., Read W. A., Castle L., Gilbert J. Levels of di-(2-ethylhexyl)phthalate and total phthalate esters in milk, cream, butter and cheese. Food Addit Contam. 1994 May-Jun;11(3):375–385. doi: 10.1080/02652039409374236. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M. Declining sperm counts in men--is there an endocrine cause? J Endocrinol. 1993 Mar;136(3):357–360. doi: 10.1677/joe.0.1360357. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Kerr J. B., McKinnell C., Millar M. Temporal relationship between androgen-dependent changes in the volume of seminiferous tubule fluid, lumen size and seminiferous tubule protein secretion in rats. J Reprod Fertil. 1994 May;101(1):193–198. doi: 10.1530/jrf.0.1010193. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Maddocks S., Millar M., Kerr J. B., Saunders P. T., McKinnell C. Testosterone and spermatogenesis. Identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J Androl. 1992 Mar-Apr;13(2):172–184. [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Singh A. R., Lawrence W. H., Autian J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J Pharm Sci. 1975 Aug;64(8):1347–1350. doi: 10.1002/jps.2600640819. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994 Apr;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Wing T. Y., Christensen A. K. Morphometric studies on rat seminiferous tubules. Am J Anat. 1982 Sep;165(1):13–25. doi: 10.1002/aja.1001650103. [DOI] [PubMed] [Google Scholar]