Abstract
Tetrachloroethene (PCE) and other chloroethenes are major contaminants in groundwater, and PCE is particularly resistant to attack by aerobes. We have developed an anaerobic enrichment culture that carries out reductive dechlorination of chloroethenes to ethene at high rates, thereby detoxifying them. Although the electron donor added to the culture is methanol, our evidence indicates that H2 is the electron donor used directly for dechlorination. We have recently obtained a culture from 10(-6) dilution of the original methanol/PCE culture that uses H2 as an electron donor for PCE dechlorination. Because the culture can be transferred indefinitely and the rate of PCE dechlorination increases after inoculation, we suggest that dechlorinating organisms in the culture use the carbon-chlorine bonds in chloroethenes as electron acceptors for energy conservation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DiStefano T. D., Gossett J. M., Zinder S. H. Hydrogen as an electron donor for dechlorination of tetrachloroethene by an anaerobic mixed culture. Appl Environ Microbiol. 1992 Nov;58(11):3622–3629. doi: 10.1128/aem.58.11.3622-3629.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
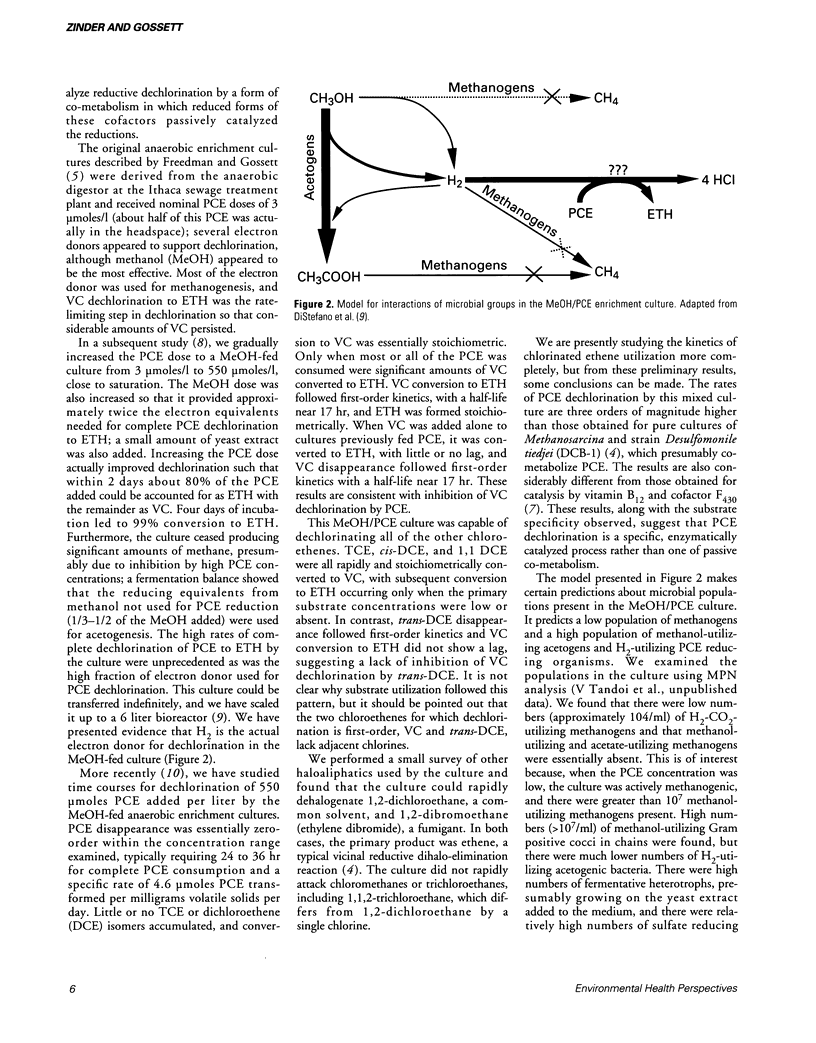
- DiStefano T. D., Gossett J. M., Zinder S. H. Reductive dechlorination of high concentrations of tetrachloroethene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Appl Environ Microbiol. 1991 Aug;57(8):2287–2292. doi: 10.1128/aem.57.8.2287-2292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D. Biochemical diversity of trichloroethylene metabolism. Annu Rev Microbiol. 1991;45:283–299. doi: 10.1146/annurev.mi.45.100191.001435. [DOI] [PubMed] [Google Scholar]
- Freedman D. L., Gossett J. M. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol. 1989 Sep;55(9):2144–2151. doi: 10.1128/aem.55.9.2144-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger C., Schraa G., Stams A. J., Zehnder A. J. A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Appl Environ Microbiol. 1993 Sep;59(9):2991–2997. doi: 10.1128/aem.59.9.2991-2997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn W. W., Tiedje J. M. Microbial reductive dehalogenation. Microbiol Rev. 1992 Sep;56(3):482–507. doi: 10.1128/mr.56.3.482-507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin W. P., Kotterman M. J., Posthumus M. A., Schraa G., Zehnder A. J. Complete biological reductive transformation of tetrachloroethene to ethane. Appl Environ Microbiol. 1992 Jun;58(6):1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


