Abstract
The cytochrome P450 enzymes are major catalysts involved in the oxidations of xenobiotic chemicals in microorganisms as well as higher animals and plants. Because of their functional roles, they offer potential in biodegradation technology. A number of microbial P450s have already been characterized and offer advantages in terms of their high catalytic rates and facile expression in microorganisms. One approach to extending the catalytic selectivity to more compounds in the environment is rational design. In three cases, the three-dimensional structures of bacterial cytochrome P450 enzymes are available and can be further understood through studies with molecular dynamics. Many mammalian cytochrome P450 enzymes have been studied extensively and have potential for biodegradation because of their broad catalytic selectivities (e.g., P450 2E1). Several advances have been made in the heterologous expression of these proteins in microorganisms. Improvements under development include electron transfer from flavodoxin and the use of cytochrome P450:NADPH-cytochrome P450 reductase fusion proteins. Random mutagenesis offers the potential of improving the catalytic activities of some of these proteins. Future challenges include the use of cytochrome P450 expression vectors in microorganisms capable of thriving in the environment; recent success in expression of vectors in Salmonella genotoxicity tester strains may be encouraging in this regard.
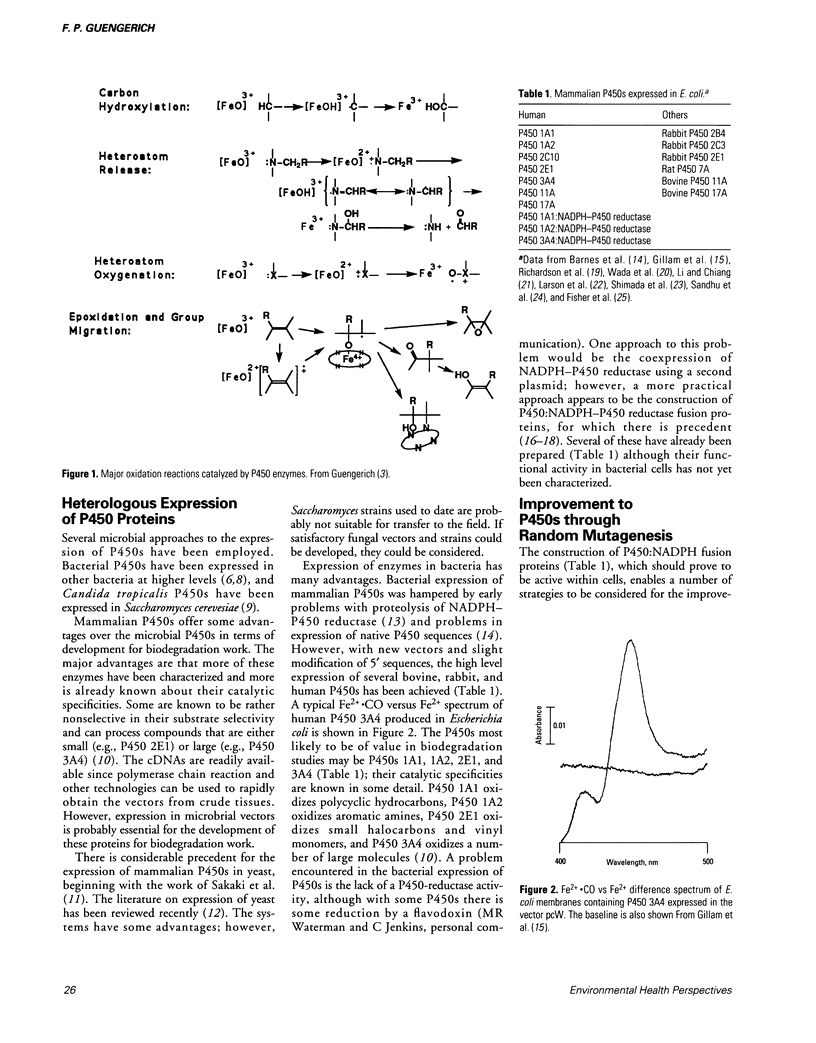
Full text
PDF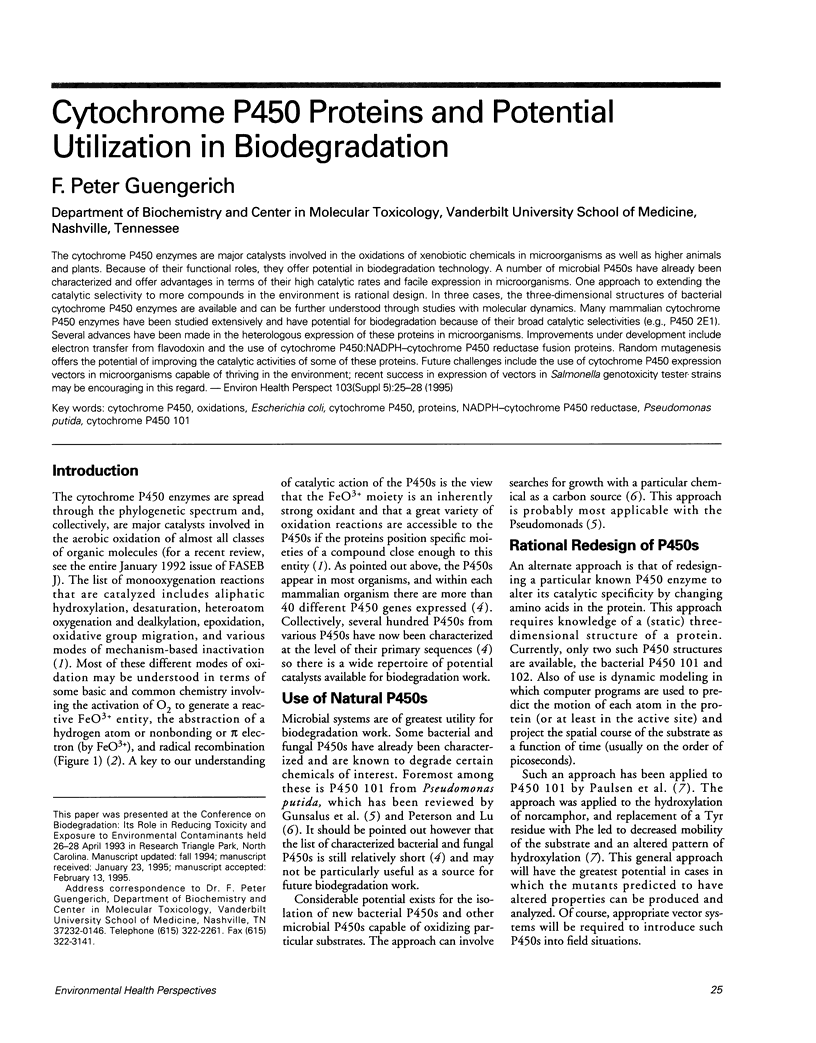


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes H. J., Arlotto M. P., Waterman M. R. Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5597–5601. doi: 10.1073/pnas.88.13.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Turi T. G., Sanglard D., Loper J. C. Isolation of the Candida tropicalis gene for P450 lanosterol demethylase and its expression in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1311–1317. doi: 10.1016/0006-291x(87)90792-3. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Loeb L. A. Mutants generated by the insertion of random oligonucleotides into the active site of the beta-lactamase gene. Biochemistry. 1989 Jul 11;28(14):5703–5707. doi: 10.1021/bi00440a001. [DOI] [PubMed] [Google Scholar]
- Fisher C. W., Shet M. S., Caudle D. L., Martin-Wixtrom C. A., Estabrook R. W. High-level expression in Escherichia coli of enzymatically active fusion proteins containing the domains of mammalian cytochromes P450 and NADPH-P450 reductase flavoprotein. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10817–10821. doi: 10.1073/pnas.89.22.10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam E. M., Baba T., Kim B. R., Ohmori S., Guengerich F. P. Expression of modified human cytochrome P450 3A4 in Escherichia coli and purification and reconstitution of the enzyme. Arch Biochem Biophys. 1993 Aug 15;305(1):123–131. doi: 10.1006/abbi.1993.1401. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Brian W. R., Sari M. A., Ross J. T. Expression of mammalian cytochrome P450 enzymes using yeast-based vectors. Methods Enzymol. 1991;206:130–145. doi: 10.1016/0076-6879(91)06085-h. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., MacDonald T. L. Mechanisms of cytochrome P-450 catalysis. FASEB J. 1990 May;4(8):2453–2459. doi: 10.1096/fasebj.4.8.2185971. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Reactions and significance of cytochrome P-450 enzymes. J Biol Chem. 1991 Jun 5;266(16):10019–10022. [PubMed] [Google Scholar]
- Guengerich F. P., Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991 Jul-Aug;4(4):391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. The 1992 Bernard B. Brodie Award Lecture. Bioactivation and detoxication of toxic and carcinogenic chemicals. Drug Metab Dispos. 1993 Jan-Feb;21(1):1–6. [PubMed] [Google Scholar]
- Gunsalus I. C., Pederson T. C., Sligar S. G. Oxygenase-catalyzed biological hydroxylations. Annu Rev Biochem. 1975;44:377–407. doi: 10.1146/annurev.bi.44.070175.002113. [DOI] [PubMed] [Google Scholar]
- Hermes J. D., Blacklow S. C., Knowles J. R. Searching sequence space by definably random mutagenesis: improving the catalytic potency of an enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):696–700. doi: 10.1073/pnas.87.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J. R., Coon M. J., Porter T. D. Alcohol-inducible cytochrome P-450IIE1 lacking the hydrophobic NH2-terminal segment retains catalytic activity and is membrane-bound when expressed in Escherichia coli. J Biol Chem. 1991 Apr 25;266(12):7321–7324. [PubMed] [Google Scholar]
- Li Y. C., Chiang J. Y. The expression of a catalytically active cholesterol 7 alpha-hydroxylase cytochrome P450 in Escherichia coli. J Biol Chem. 1991 Oct 15;266(29):19186–19191. [PubMed] [Google Scholar]
- Murakami H., Yabusaki Y., Sakaki T., Shibata M., Ohkawa H. A genetically engineered P450 monooxygenase: construction of the functional fused enzyme between rat cytochrome P450c and NADPH-cytochrome P450 reductase. DNA. 1987 Jun;6(3):189–197. doi: 10.1089/dna.1987.6.189. [DOI] [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986 Jun 5;261(16):7160–7169. [PubMed] [Google Scholar]
- Nelson D. R., Kamataki T., Waxman D. J., Guengerich F. P., Estabrook R. W., Feyereisen R., Gonzalez F. J., Coon M. J., Gunsalus I. C., Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993 Jan-Feb;12(1):1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Paulsen M. D., Filipovic D., Sligar S. G., Ornstein R. L. Controlling the regiospecificity and coupling of cytochrome P450cam: T185F mutant increases coupling and abolishes 3-hydroxynorcamphor product. Protein Sci. 1993 Mar;2(3):357–365. doi: 10.1002/pro.5560020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. A., Lu J. Y. Bacterial cytochromes P450: isolation and identification. Methods Enzymol. 1991;206:612–620. doi: 10.1016/0076-6879(91)06131-l. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Wilson T. E., Kasper C. B. Expression of a functional 78,000 dalton mammalian flavoprotein, NADPH-cytochrome P-450 oxidoreductase, in Escherichia coli. Arch Biochem Biophys. 1987 Apr;254(1):353–367. doi: 10.1016/0003-9861(87)90111-1. [DOI] [PubMed] [Google Scholar]
- Richardson T. H., Hsu M. H., Kronbach T., Barnes H. J., Chan G., Waterman M. R., Kemper B., Johnson E. F. Purification and characterization of recombinant-expressed cytochrome P450 2C3 from Escherichia coli: 2C3 encodes the 6 beta-hydroxylase deficient form of P450 3b. Arch Biochem Biophys. 1993 Jan;300(1):510–516. doi: 10.1006/abbi.1993.1069. [DOI] [PubMed] [Google Scholar]
- Sakaki T., Oeda K., Miyoshi M., Ohkawa H. Characterization of rat cytochrome P-450MC synthesized in Saccharomyces cerevisiae. J Biochem. 1985 Jul;98(1):167–175. doi: 10.1093/oxfordjournals.jbchem.a135255. [DOI] [PubMed] [Google Scholar]
- Sandhu P., Baba T., Guengerich F. P. Expression of modified cytochrome P450 2C10 (2C9) in Escherichia coli, purification, and reconstitution of catalytic activity. Arch Biochem Biophys. 1993 Nov 1;306(2):443–450. doi: 10.1006/abbi.1993.1536. [DOI] [PubMed] [Google Scholar]
- Shimada T., Gillam E. M., Sandhu P., Guo Z., Tukey R. H., Guengerich F. P. Activation of procarcinogens by human cytochrome P450 enzymes expressed in Escherichia coli. Simplified bacterial systems for genotoxicity assays. Carcinogenesis. 1994 Nov;15(11):2523–2529. doi: 10.1093/carcin/15.11.2523. [DOI] [PubMed] [Google Scholar]
- Unger B. P., Gunsalus I. C., Sligar S. G. Nucleotide sequence of the Pseudomonas putida cytochrome P-450cam gene and its expression in Escherichia coli. J Biol Chem. 1986 Jan 25;261(3):1158–1163. [PubMed] [Google Scholar]
- Wada A., Mathew P. A., Barnes H. J., Sanders D., Estabrook R. W., Waterman M. R. Expression of functional bovine cholesterol side chain cleavage cytochrome P450 (P450scc) in Escherichia coli. Arch Biochem Biophys. 1991 Nov 1;290(2):376–380. doi: 10.1016/0003-9861(91)90554-v. [DOI] [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]


