Abstract
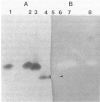
Several anaerobic bacteria from the human intestinal tract are capable of reducing azo dyes and nitropolycyclic aromatic hydrocarbons to the corresponding aromatic amines with enzymes that have azoreductase and nitroreductase activities. The majority of bacteria with these activities belong to the genera Clostridium and Eubacterium. The azoreductases and nitroreductases from three Clostridium strains and one Eubacterium strain were studied. Both enzymes were produced constitutively in each of the bacteria; the enzymes from various bacteria had different electrophoretic mobilities. The azoreductases from all of the bacteria had immunological homology, as was evident from the cross-reactivity of an antibody raised against the azoreductase of C. perfringens with azoreductases from other bacteria. Comparison of azoreductases and nitroreductases showed that they both had identical electrophoretic mobilities on polyacrylamide gels and reacted with the antibody against the azoreductase from C. perfringens. Furthermore, the nitroaromatic compounds competitively inhibited the azoreductase activity. The data indicate that the reduction of both nitroaromatic compounds and azo dyes may be carried out by the same enzyme, which is possibly a flavin adenine dinucleotide dehydrogenase that is synthesized throughout the cell and not associated with any organized subcellular structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerniglia C. E., Freeman J. P., Franklin W., Pack L. D. Metabolism of azo dyes derived from benzidine, 3,3'-dimethyl-benzidine and 3,3'-dimethoxybenzidine to potentially carcinogenic aromatic amines by intestinal bacteria. Carcinogenesis. 1982;3(11):1255–1260. doi: 10.1093/carcin/3.11.1255. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E. Metabolism of 1-nitropyrene and 6-nitrobenzo(a)pyrene by intestinal microflora. Prog Clin Biol Res. 1985;181:133–137. [PubMed] [Google Scholar]
- Fujita S., Peisach J. The stimulation of microsomal azoreduction by flavins. Biochim Biophys Acta. 1982 Nov 24;719(2):178–189. doi: 10.1016/0304-4165(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Gingell R., Walker R. Mechanisms of azo reduction by Streptococcus faecalis. II. The role of soluble flavins. Xenobiotica. 1971 May;1(3):231–239. doi: 10.3109/00498257109033172. [DOI] [PubMed] [Google Scholar]
- Howard P. C., Beland F. A., Cerniglia C. E. Reduction of the carcinogen 1-nitropyrene to 1-aminopyrene by rat intestinal bacteria. Carcinogenesis. 1983 Aug;4(8):985–990. doi: 10.1093/carcin/4.8.985. [DOI] [PubMed] [Google Scholar]
- Levine W. G., Raza H. Mechanism of azoreduction of dimethylaminoazobenzene by rat liver NADPH-cytochrome P-450 reductase and partially purified cytochrome P-450. Oxygen and carbon monoxide sensitivity and stimulation by FAD and FMN. Drug Metab Dispos. 1988 May-Jun;16(3):441–448. [PubMed] [Google Scholar]
- Manning B. W., Campbell W. L., Franklin W., Delclos K. B., Cerniglia C. E. Metabolism of 6-nitrochrysene by intestinal microflora. Appl Environ Microbiol. 1988 Jan;54(1):197–203. doi: 10.1128/aem.54.1.197-203.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii F., Cerniglia C. E. Comparison of the azoreductase and nitroreductase from Clostridium perfringens. Appl Environ Microbiol. 1993 Jun;59(6):1731–1734. doi: 10.1128/aem.59.6.1731-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii F., Franklin W., Cerniglia C. E. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol. 1990 Jul;56(7):2146–2151. doi: 10.1128/aem.56.7.2146-2151.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii F., Smith D. B., Benson R. W., Cerniglia C. E. Immunological homology among azoreductases from Clostridium and Eubacterium strains isolated from human intestinal microflora. J Basic Microbiol. 1992;32(2):99–105. doi: 10.1002/jobm.3620320204. [DOI] [PubMed] [Google Scholar]
- Rafil F., Franklin W., Heflich R. H., Cerniglia C. E. Reduction of nitroaromatic compounds by anaerobic bacteria isolated from the human gastrointestinal tract. Appl Environ Microbiol. 1991 Apr;57(4):962–968. doi: 10.1128/aem.57.4.962-968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariah P. K., Juchau M. R. The role of gut flora in the reduction of aromatic nitro-groups. Drug Metab Dispos. 1974 Jan-Feb;2(1):74–78. [PubMed] [Google Scholar]