Abstract
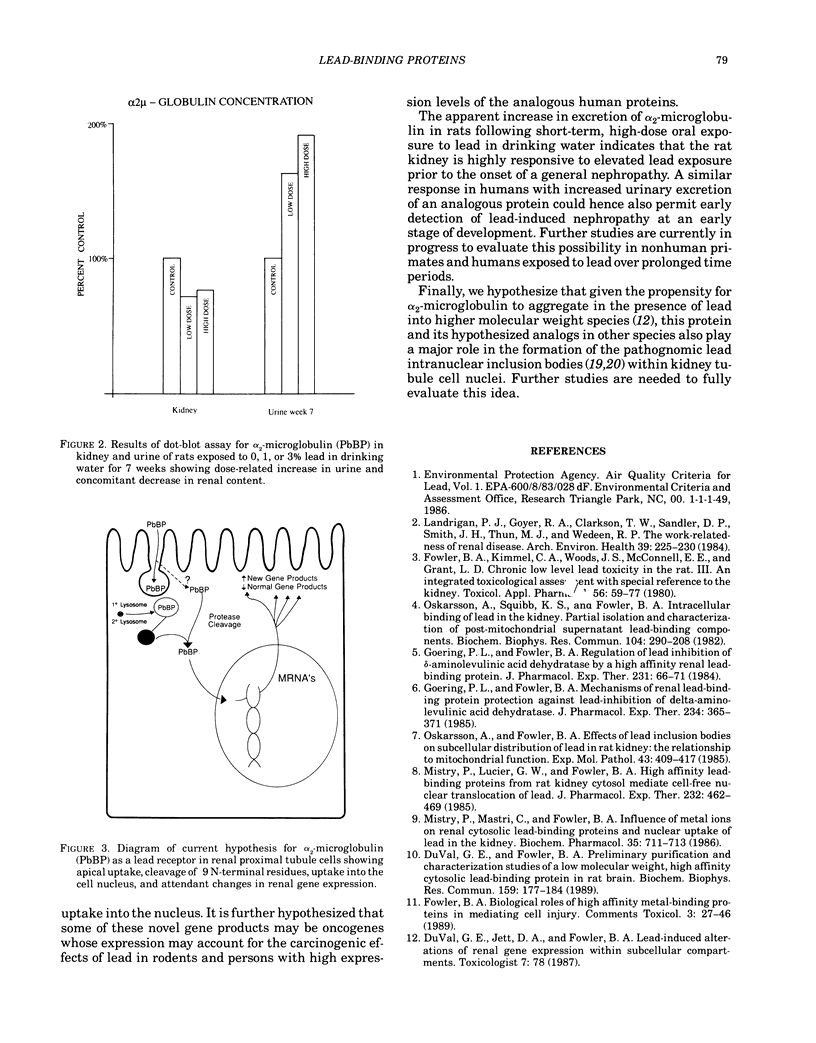
Lead-induced nephropathy produces both tubular and interstitial manifestations of cell injury, but the pathophysiology of these lesions is not completely understood. Delineation of the molecular factors underlying renal handling of lead is one of central importance in understanding the mechanisms of renal cell injury from this agent. Recent studies from this laboratory have identified several distinct high-affinity lead-binding proteins (PbBP) from rat kidney and brain that appear to play critical roles in the intracellular bioavailability of lead to several essential cellular processes in these target tissues at low dose levels. The PbBP from rat kidney has been shown to be a specific cleavage product of alpha 2-microglobulin, which is a member of the retinol-binding protein superfamily. Recent preliminary Western blot and immunohistochemical studies have shown that a polyclonal antibody to the renal PbBP does not recognize the brain PbBP, which appears to be a chemically similar, but distinct molecule. These studies have also shown that the renal PbBP is selectively localized in only certain nephrons and only specific segments of the renal proximal tubule. The striking nephror and cell-type specificity of the localization reaction could result from physiological differences in nephron functional activity or selective molecular uptake mechanisms/metabolism differences that act to define target cell populations in the kidney. In addition, other preliminary studies have shown that short-term, high-dose lead exposure produces increased excretion of this protein into the urine with concomitant decreases in renal concentrations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Domin B. A., Serabjit-Singh C. J., Philpot R. M. Quantitation of rabbit cytochrome P-450, form 2, in microsomal preparations bound directly to nitrocellulose paper using a modified peroxidase-immunostaining procedure. Anal Biochem. 1984 Feb;136(2):390–396. doi: 10.1016/0003-2697(84)90234-3. [DOI] [PubMed] [Google Scholar]
- DuVal G., Fowler B. A. Preliminary purification and characterization studies of a low molecular weight, high affinity cytosolic lead-binding protein in rat brain. Biochem Biophys Res Commun. 1989 Feb 28;159(1):177–184. doi: 10.1016/0006-291x(89)92420-0. [DOI] [PubMed] [Google Scholar]
- Fowler B. A., Kimmel C. A., Woods J. S., McConnell E. E., Grant L. D. Chronic low-level lead toxicity in the rat. III. An integrated assessment of long-term toxicity with special reference to the kidney. Toxicol Appl Pharmacol. 1980 Oct;56(1):59–77. doi: 10.1016/0041-008x(80)90131-3. [DOI] [PubMed] [Google Scholar]
- Goering P. L., Fowler B. A. Mechanism of renal lead-binding protein reversal of delta-aminolevulinic acid dehydratase inhibition by lead. J Pharmacol Exp Ther. 1985 Aug;234(2):365–371. [PubMed] [Google Scholar]
- Goering P. L., Fowler B. A. Regulation of lead inhibition of delta-aminolevulinic acid dehydratase by a low molecular weight, high affinity renal lead-binding protein. J Pharmacol Exp Ther. 1984 Oct;231(1):66–71. [PubMed] [Google Scholar]
- Landrigan P. J., Goyer R. A., Clarkson T. W., Sandler D. P., Smith J. H., Thun M. J., Wedeen R. P. The work-relatedness of renal disease. Arch Environ Health. 1984 May-Jun;39(3):225–230. doi: 10.1080/00039896.1984.9939529. [DOI] [PubMed] [Google Scholar]
- Mistry P., Lucier G. W., Fowler B. A. High-affinity lead binding proteins in rat kidney cytosol mediate cell-free nuclear translocation of lead. J Pharmacol Exp Ther. 1985 Feb;232(2):462–469. [PubMed] [Google Scholar]
- Mistry P., Mastri C., Fowler B. A. Influence of metal ions on renal cytosolic lead-binding proteins and nuclear uptake of lead in the kidney. Biochem Pharmacol. 1986 Feb 15;35(4):711–713. doi: 10.1016/0006-2952(86)90371-0. [DOI] [PubMed] [Google Scholar]
- Moore J. F., Goyer R. A., Wilson M. Lead-induced inclusion bodies. Solubility, amino acid content, and relationship to residual acidic nuclear proteins. Lab Invest. 1973 Nov;29(5):488–494. [PubMed] [Google Scholar]
- Murakami M., Kawamura R., Nishii S., Katsunuma H. Early appearance and localization of intranuclear inclusions in the segments of renal proximal tubules of rats following ingestion of lead. Br J Exp Pathol. 1983 Apr;64(2):144–155. [PMC free article] [PubMed] [Google Scholar]
- Oskarsson A., Fowler B. A. Effects of lead on the heme biosynthetic pathway in rat kidney. Exp Mol Pathol. 1985 Dec;43(3):409–417. doi: 10.1016/0014-4800(85)90077-2. [DOI] [PubMed] [Google Scholar]
- Oskarsson A., Squibb K. S., Fowler B. A. Intracellular binding of lead in the kidney: the partial isolation and characterization of postmitochondrial lead binding components. Biochem Biophys Res Commun. 1982 Jan 15;104(1):290–298. doi: 10.1016/0006-291x(82)91973-8. [DOI] [PubMed] [Google Scholar]
- Roy A. K., Raber D. L. Immunofluorescent localization of 2u -globulin in the hepatic and renal tissues of rat. J Histochem Cytochem. 1972 Feb;20(2):89–96. doi: 10.1177/20.2.89. [DOI] [PubMed] [Google Scholar]
- Swenberg J. A., Short B., Borghoff S., Strasser J., Charbonneau M. The comparative pathobiology of alpha 2u-globulin nephropathy. Toxicol Appl Pharmacol. 1989 Jan;97(1):35–46. doi: 10.1016/0041-008x(89)90053-7. [DOI] [PubMed] [Google Scholar]



