Abstract
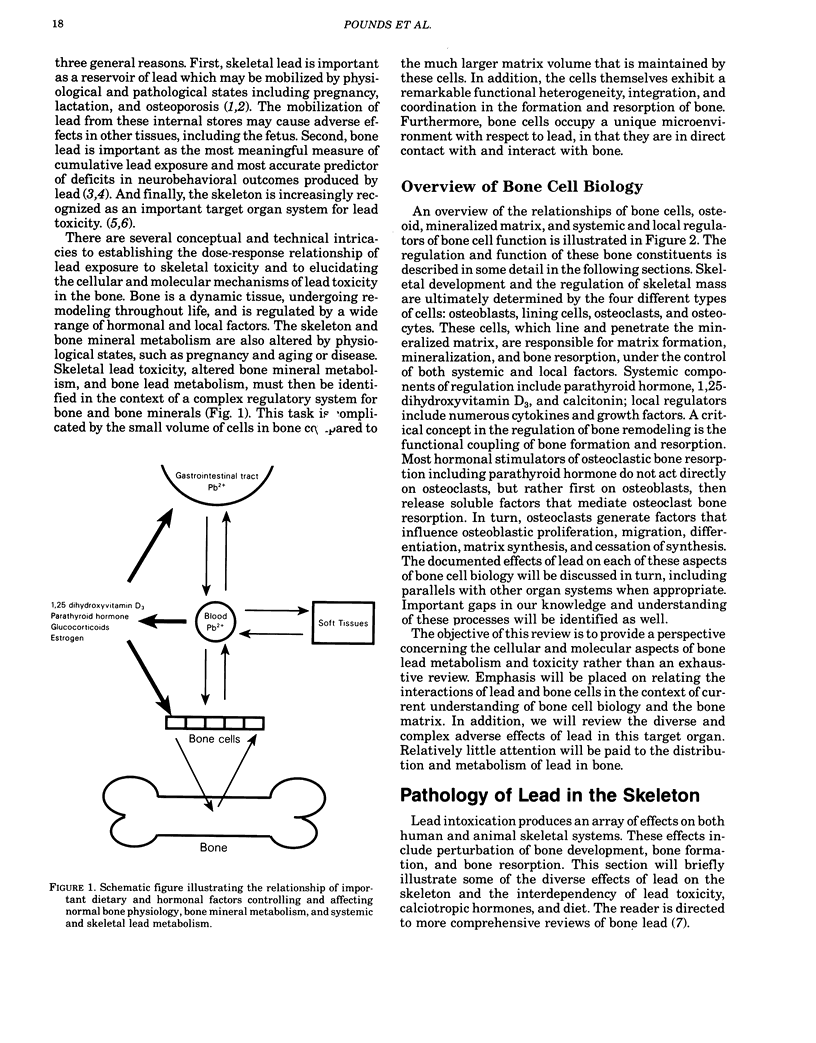
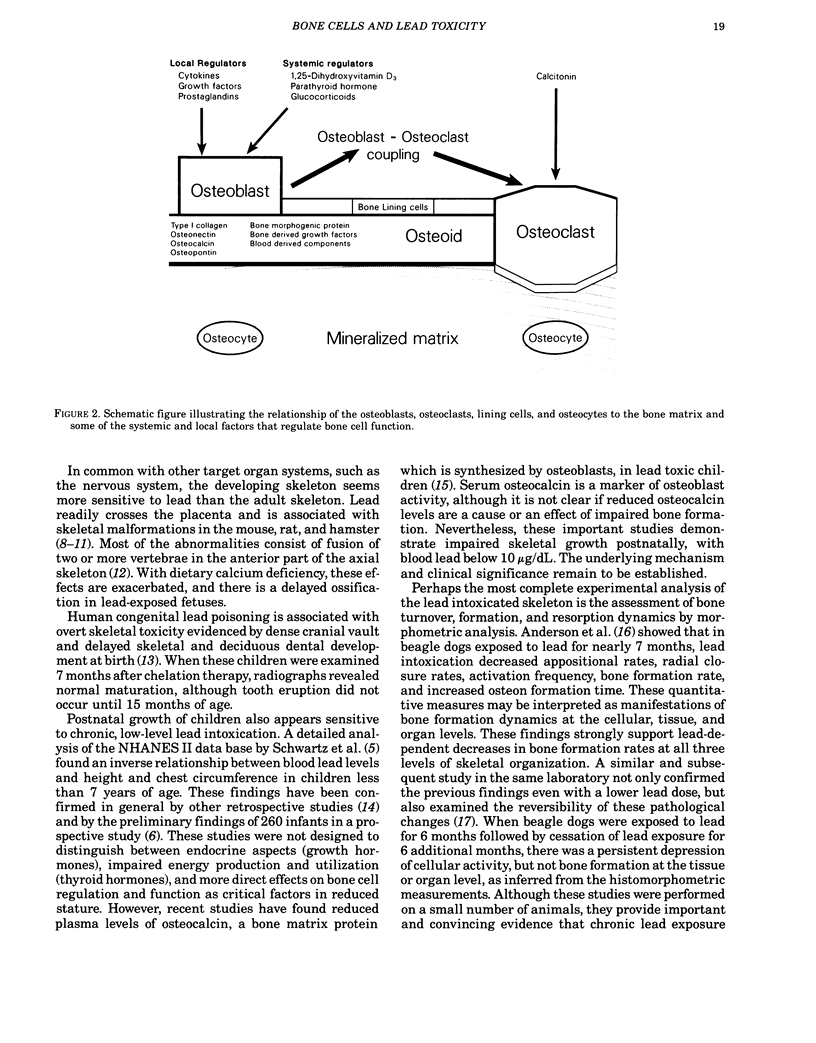
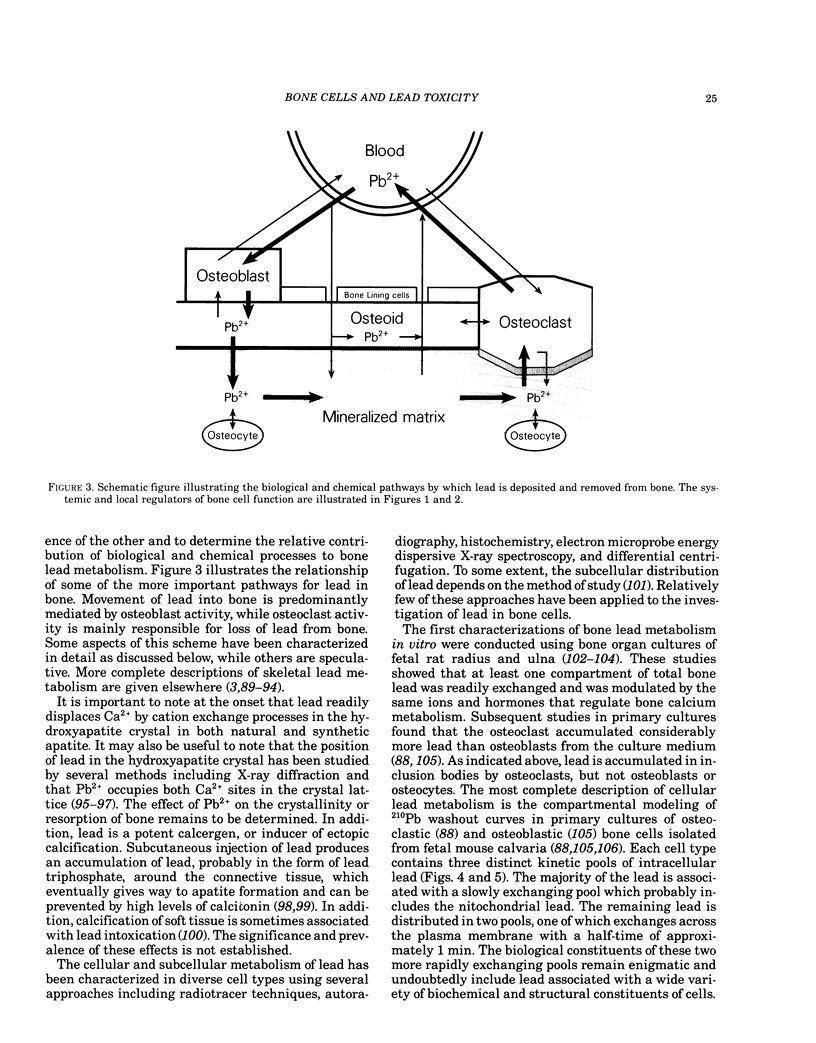
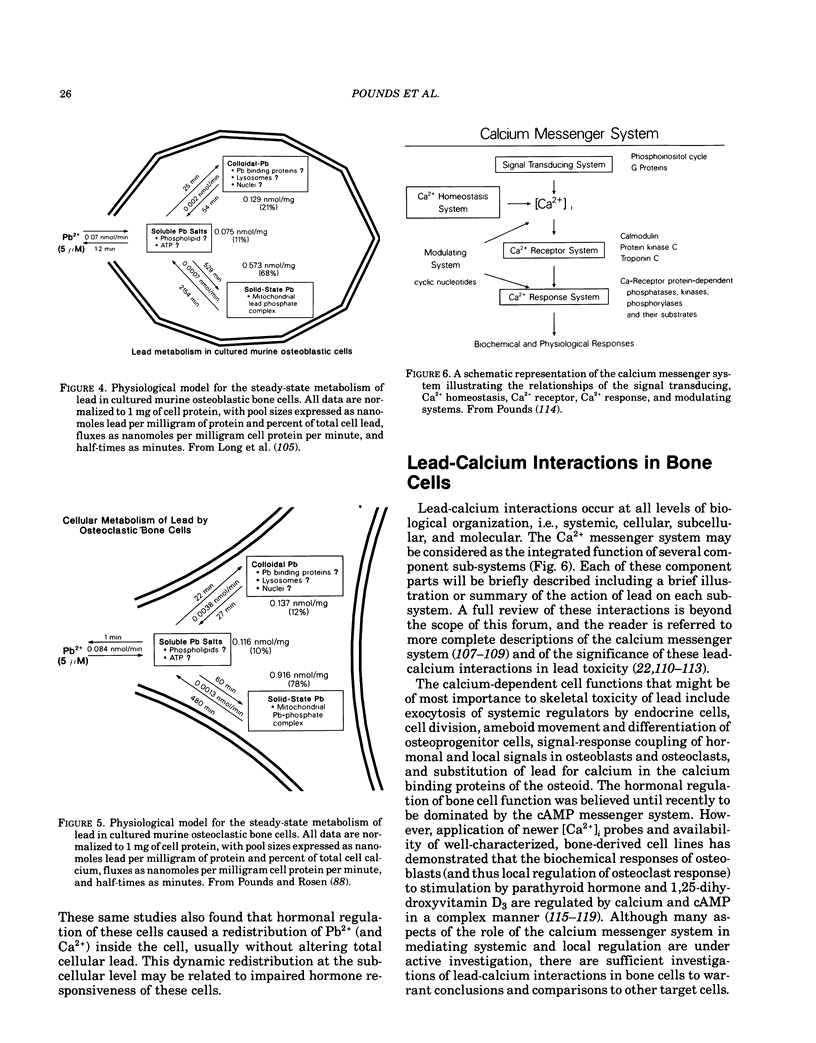
To fully understand the significance of bone as a target tissue of lead toxicity, as well as a reservoir of systemic lead, it is necessary to define the effects of lead on the cellular components of bone. Skeletal development and the regulation of skeletal mass are ultimately determined by the four different types of cells: osteoblasts, lining cells, osteoclasts, and osteocytes. These cells, which line and penetrate the mineralized matrix, are responsible for matrix formation, mineralization, and bone resorption, under the control of both systemic and local factors. Systemic components of regulation include parathyroid hormone, 1,25-dihydroxyvitamin D3, and calcitonin: local regulators include numerous cytokines and growth factors. Lead intoxication directly and indirectly alters many aspects of bone cell function. First, lead may indirectly alter bone cell function through changes in the circulating levels of those hormones, particularly 1,25-dihydroxyvitamin D3, which modulate bone cell function. These hormonal changes have been well established in clinical studies, although the functional significance remains to be established. Second, lead may directly alter bone cell function by perturbing the ability of bone cells to respond to hormonal regulation. For example, the 1,25-dihydroxyvitamin D3-stimulated synthesis of osteocalcin, a calcium-binding protein synthesized by osteoblastic bone cells, is inhibited by low levels of lead. Impaired osteocalcin production may inhibit new bone formation, as well as the functional coupling of osteoblasts and osteoclasts. Third, lead may impair the ability of cells to synthesize or secrete other components of the bone matrix, such as collagen or bone sialoproteins (osteopontin). Finally, lead may directly effect or substitute for calcium in the active sites of the calcium messenger system, resulting in loss of physiological regulation. The effects of lead on the recruitment and differentiation of bone cells remains to be established. Compartmental analysis indicates that the kinetic distribution and behavior of intracellular lead in osteoblasts and osteoclasts is similar to several other cell types. Many of the toxic effects of lead on bone cell function may be produced by perturbation of the calcium and cAMP messenger systems in these cells.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Anderson C., Danylchuk K. D. Haversian bone-remodelling rates in the beagle after cessation of exposure to chronic low doses of lead. J Environ Pathol Toxicol. 1980 Jun-Jul;3(5-6):413–422. [PubMed] [Google Scholar]
- Anderson C., Danylchuk K. D. The effect of chronic low level lead intoxication on the Haversian remodeling system in dogs. Lab Invest. 1977 Nov;37(5):466–469. [PubMed] [Google Scholar]
- Anderson R. E., Schraer H., Gay C. V. Ultrastructural immunocytochemical localization of carbonic anhydrase in normal and calcitonin-treated chick osteoclasts. Anat Rec. 1982 Sep;204(1):9–20. doi: 10.1002/ar.1092040103. [DOI] [PubMed] [Google Scholar]
- Audesirk G. Effects of lead exposure on the physiology of neurons. Prog Neurobiol. 1985;24(3):199–231. doi: 10.1016/0301-0082(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Bennett J., Weeds A. Calcium and the cytoskeleton. Br Med Bull. 1986 Oct;42(4):385–390. doi: 10.1093/oxfordjournals.bmb.a072156. [DOI] [PubMed] [Google Scholar]
- Bertoni J. M., Sprenkle P. M. Inhibition of brain cation pump enzyme by in vitro lead ion: effects of low level [Pb] and modulation by homogenate. Toxicol Appl Pharmacol. 1988 Mar 30;93(1):101–107. doi: 10.1016/0041-008x(88)90029-4. [DOI] [PubMed] [Google Scholar]
- Bonucci E., Barckhaus R. H., Silvestrini G., Ballanti P., Di Lorenzo G. Osteoclast changes induced by lead poisoning (saturnism). Appl Pathol. 1983;1(5):241–250. [PubMed] [Google Scholar]
- Bonucci E. New knowledge on the origin, function and fate of osteoclasts. Clin Orthop Relat Res. 1981 Jul-Aug;(158):252–269. [PubMed] [Google Scholar]
- Bonucci E., Silvestrini G. Ultrastructural studies in experimental lead intoxication. Contrib Nephrol. 1988;64:93–101. doi: 10.1159/000415731. [DOI] [PubMed] [Google Scholar]
- Bonucci E. The organic-inorganic relationships in bone matrix undergoing osteoclastic resorption. Calcif Tissue Res. 1974;16(1):13–36. doi: 10.1007/BF02008210. [DOI] [PubMed] [Google Scholar]
- Breen K. C., Regan C. M. Lead stimulates Golgi sialyltransferase at times coincident with the embryonic to adult conversion of the neural cell adhesion molecule (N-CAM). Toxicology. 1988 Apr;49(1):71–76. doi: 10.1016/0300-483x(88)90176-x. [DOI] [PubMed] [Google Scholar]
- Bushnell P. J., Shelton S. E., Bowman R. E. Elevation of blood lead concentration by confinement in the rhesus monkey. Bull Environ Contam Toxicol. 1979 Aug;22(6):819–826. doi: 10.1007/BF02027031. [DOI] [PubMed] [Google Scholar]
- Calhoun L. A., Livesey D. L., Mailer K., Addetia R. Interaction of lead ions with bovine carbonic anhydrase: further studies. J Inorg Biochem. 1985 Dec;25(4):261–275. doi: 10.1016/0162-0134(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. The pathobiology of the osteoclast. J Clin Pathol. 1985 Mar;38(3):241–252. doi: 10.1136/jcp.38.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L. G., Fox D. A. A selective decrease of cholinergic muscarinic receptors in the visual cortex of adult rats following developmental lead exposure. Brain Res. 1983 Oct 16;276(2):259–266. doi: 10.1016/0006-8993(83)90733-3. [DOI] [PubMed] [Google Scholar]
- Dedman J. R. Mediation of intracellular calcium: variances on a common theme. Cell Calcium. 1986 Dec;7(5-6):297–307. doi: 10.1016/0143-4160(86)90034-5. [DOI] [PubMed] [Google Scholar]
- Delaquerriere-Richardson L., Anderson C., Jorch U. M., Cook M. Radiographic studies on bone in beagles subjected to low levels of dietary lead since birth. Vet Hum Toxicol. 1982 Dec;24(6):401–405. [PubMed] [Google Scholar]
- Epstein S. Serum and urinary markers of bone remodeling: assessment of bone turnover. Endocr Rev. 1988 Nov;9(4):437–449. doi: 10.1210/edrv-9-4-437. [DOI] [PubMed] [Google Scholar]
- Ferm V. H., Carpenter S. J. Developmental malformations resulting from the administration of lead salts. Exp Mol Pathol. 1967 Oct;7(2):208–213. doi: 10.1016/0014-4800(67)90030-5. [DOI] [PubMed] [Google Scholar]
- Ferm V. H., Ferm D. W. The specificity of the teratogenic effect of lead in the golden hamster. Life Sci II. 1971 Jan 8;10(1):35–39. doi: 10.1016/0024-3205(71)90222-0. [DOI] [PubMed] [Google Scholar]
- Fowler B. A. Mechanisms of metal-induced renal cell injury: roles of high-affinity metal-binding proteins. Contrib Nephrol. 1988;64:83–92. doi: 10.1159/000415730. [DOI] [PubMed] [Google Scholar]
- Frenkel G. D., Middleton C. Effects of lead acetate on DNA and RNA synthesis by intact HeLa cells, isolated nuclei and purified polymerases. Biochem Pharmacol. 1987 Jan 15;36(2):265–268. doi: 10.1016/0006-2952(87)90699-x. [DOI] [PubMed] [Google Scholar]
- Fritsch J., Edelman A., Balsan S. Early effects of parathyroid hormone on membrane potential of rat osteoblasts in culture: role of cAMP and Ca2+. J Bone Miner Res. 1988 Oct;3(5):547–554. doi: 10.1002/jbmr.5650030511. [DOI] [PubMed] [Google Scholar]
- Fullmer C. S., Edelstein S., Wasserman R. H. Lead-binding properties of intestinal calcium-binding proteins. J Biol Chem. 1985 Jun 10;260(11):6816–6819. [PubMed] [Google Scholar]
- Gabbiani G., Tuchweber B., Perrault G. Studies in the mechanism of metal-induced soft tissue calcification. Calcif Tissue Res. 1970;6(1):20–31. doi: 10.1007/BF02196181. [DOI] [PubMed] [Google Scholar]
- Goering P. L., Fowler B. A. Mechanism of renal lead-binding protein reversal of delta-aminolevulinic acid dehydratase inhibition by lead. J Pharmacol Exp Ther. 1985 Aug;234(2):365–371. [PubMed] [Google Scholar]
- Goering P. L., Mistry P., Fowler B. A. A low molecular weight lead-binding protein in brain attenuates lead inhibition of delta-aminolevulinic acid dehydratase: comparison with a renal lead-binding protein. J Pharmacol Exp Ther. 1986 Apr;237(1):220–225. [PubMed] [Google Scholar]
- Goldberg R. L., Kaplan S. R., Fuller G. C. Effect of heavy metals on human rheumatoid synovial cell proliferation and collagen synthesis. Biochem Pharmacol. 1983 Sep 15;32(18):2763–2766. doi: 10.1016/0006-2952(83)90089-8. [DOI] [PubMed] [Google Scholar]
- Gotti C., Cabrini D., Sher E., Clementi F. Effects of long-term in vitro exposure to aluminum, cadmium or lead on differentiation and cholinergic receptor expression in a human neuroblastoma cell line. Cell Biol Toxicol. 1987 Dec;3(4):431–440. doi: 10.1007/BF00119915. [DOI] [PubMed] [Google Scholar]
- HASS G. M., BROWN D. V., EISENSTEIN R., HEMMENS A. RELATIONS BETWEEN LEAD POISONING IN RABBIT AND MAN. Am J Pathol. 1964 Nov;45:691–727. [PMC free article] [PubMed] [Google Scholar]
- Habermann E., Crowell K., Janicki P. Lead and other metals can substitute for Ca2+ in calmodulin. Arch Toxicol. 1983 Sep;54(1):61–70. doi: 10.1007/BF00277816. [DOI] [PubMed] [Google Scholar]
- Hahn T. J., Westbrook S. L., Halstead L. R. Cortisol modulation of osteoblast metabolic activity in cultured neonatal rat bone. Endocrinology. 1984 May;114(5):1864–1870. doi: 10.1210/endo-114-5-1864. [DOI] [PubMed] [Google Scholar]
- Hamir A. N., Sullivan N. D., Handson P. D. Acid-fast inclusions in osteoclasts of a lead poisoned dog. Vet Rec. 1983 May 21;112(21):503–503. doi: 10.1136/vr.112.21.503. [DOI] [PubMed] [Google Scholar]
- Hass G. M., Landerholm W., Hemmens A. Inhibition of intercellular matrix synthesis during ingestion of inorganic lead. Am J Pathol. 1967 May;50(5):815–847. [PMC free article] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., McGuinness T. L., Huganir R. L., Greengard P. Role of protein phosphorylation in neuronal signal transduction. FASEB J. 1989 Mar;3(5):1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Herrmann-Erlee M. P., van der Meer J. M., Löwik C. W., van Leeuwen J. P., Boonekamp P. M. Different roles for calcium and cyclic AMP in the action of PTH: studies in bone explants and isolated bone cells. Bone. 1988;9(2):93–100. doi: 10.1016/8756-3282(88)90109-3. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Holtzman D., DeVries C., Nguyen H., Olson J., Bensch K. Maturation of resistance to lead encephalopathy: cellular and subcellular mechanisms. Neurotoxicology. 1984 Fall;5(3):97–124. [PubMed] [Google Scholar]
- Hsu F. S., Krook L., Shively J. N., Duncan J. R., Pond W. G. Lead inclusion bodies in osteoclasts. Science. 1973 Aug 3;181(4098):447–448. doi: 10.1126/science.181.4098.447. [DOI] [PubMed] [Google Scholar]
- Jacquet P., Gerber G. B., Leonard A., Maes J. Plasma hormone levels in normal and lead-treated pregnant mice. Experientia. 1977 Oct 15;33(10):1375–1377. doi: 10.1007/BF01920190. [DOI] [PubMed] [Google Scholar]
- Jandinski J. J. Osteoclast activating factor is now interleukin-1 beta: historical perspective and biological implications. J Oral Pathol. 1988 Apr;17(4):145–152. doi: 10.1111/j.1600-0714.1988.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Keller C. A., Doherty R. A. Bone lead mobilization in lactating mice and lead transfer to suckling offspring. Toxicol Appl Pharmacol. 1980 Sep 15;55(2):220–228. doi: 10.1016/0041-008x(80)90083-6. [DOI] [PubMed] [Google Scholar]
- Kober T. E., Cooper G. P. Lead competitively inhibits calcium-dependent synaptic transmission in the bullfrog sympathetic ganglion. Nature. 1976 Aug 19;262(5570):704–705. doi: 10.1038/262704a0. [DOI] [PubMed] [Google Scholar]
- Kowolenko M., Tracy L., Mudzinski S., Lawrence D. A. Effect of lead on macrophage function. J Leukoc Biol. 1988 Apr;43(4):357–364. doi: 10.1002/jlb.43.4.357. [DOI] [PubMed] [Google Scholar]
- Lauwers M. C., Hauspie R. C., Susanne C., Verheyden J. Comparison of biometric data of children with high and low levels of lead in the blood. Am J Phys Anthropol. 1986 Jan;69(1):107–116. doi: 10.1002/ajpa.1330690112. [DOI] [PubMed] [Google Scholar]
- Lieberherr M. Effects of vitamin D3 metabolites on cytosolic free calcium in confluent mouse osteoblasts. J Biol Chem. 1987 Sep 25;262(27):13168–13173. doi: 10.1515/9783110846713.769. [DOI] [PubMed] [Google Scholar]
- Long G. J., Rosen J. F., Pounds J. G. Cellular lead toxicity and metabolism in primary and clonal osteoblastic bone cells. Toxicol Appl Pharmacol. 1990 Feb;102(2):346–361. doi: 10.1016/0041-008x(90)90032-p. [DOI] [PubMed] [Google Scholar]
- Mahaffey K. R., Rader J. I. Metabolic interactions: lead, calcium, and iron. Ann N Y Acad Sci. 1980;355:285–297. [PubMed] [Google Scholar]
- Mahaffey K. R., Rosen J. F., Chesney R. W., Peeler J. T., Smith C. M., DeLuca H. F. Association between age, blood lead concentration, and serum 1,25-dihydroxycholecalciferol levels in children. Am J Clin Nutr. 1982 Jun;35(6):1327–1331. doi: 10.1093/ajcn/35.6.1327. [DOI] [PubMed] [Google Scholar]
- Mailer K., Calhoun L. A., Livesey D. L. Interaction of lead ions with bovine carbonic anhydrase. Int J Pept Protein Res. 1982 Mar;19(3):233–239. doi: 10.1111/j.1399-3011.1982.tb03031.x. [DOI] [PubMed] [Google Scholar]
- Marcus A. H. Multicompartment kinetic model for lead. III. Lead in blood plasma and erythrocytes. Environ Res. 1985 Apr;36(2):473–489. doi: 10.1016/0013-9351(85)90039-8. [DOI] [PubMed] [Google Scholar]
- Marcus A. H. Multicompartment kinetic models for lead. I. Bone diffusion models for long-term retention. Environ Res. 1985 Apr;36(2):441–458. doi: 10.1016/0013-9351(85)90037-4. [DOI] [PubMed] [Google Scholar]
- Marcus A. H. Multicompartment kinetic models for lead. II. Linear kinetics and variable absorption in humans without excessive lead exposures. Environ Res. 1985 Apr;36(2):459–472. doi: 10.1016/0013-9351(85)90038-6. [DOI] [PubMed] [Google Scholar]
- Marcus A. H. The body burden of lead: comparison of mathematical models for accumulation. Environ Res. 1979 Jun;19(1):79–90. doi: 10.1016/0013-9351(79)90036-7. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Popoff S. N. Bone cell biology: the regulation of development, structure, and function in the skeleton. Am J Anat. 1988 Sep;183(1):1–44. doi: 10.1002/aja.1001830102. [DOI] [PubMed] [Google Scholar]
- Maytin E. V., Young D. A. Separate glucocorticoid, heavy metal, and heat shock domains in thymic lymphocytes. J Biol Chem. 1983 Oct 25;258(20):12718–12722. [PubMed] [Google Scholar]
- McCulloch C. A., Heersche J. N. Lifetime of the osteoblast in mouse periodontium. Anat Rec. 1988 Oct;222(2):128–135. doi: 10.1002/ar.1092220204. [DOI] [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. Osteoblast-like cells in the presence of parathyroid hormone release soluble factor that stimulates osteoclastic bone resorption. Endocrinology. 1986 Oct;119(4):1654–1659. doi: 10.1210/endo-119-4-1654. [DOI] [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. Endocrinology. 1986 Feb;118(2):824–828. doi: 10.1210/endo-118-2-824. [DOI] [PubMed] [Google Scholar]
- Mistry P., Lucier G. W., Fowler B. A. High-affinity lead binding proteins in rat kidney cytosol mediate cell-free nuclear translocation of lead. J Pharmacol Exp Ther. 1985 Feb;232(2):462–469. [PubMed] [Google Scholar]
- Mittelstaedt R. A., Pounds J. G. Subcellular distribution of lead in cultured rat hepatocytes. Environ Res. 1984 Oct;35(1):188–196. doi: 10.1016/0013-9351(84)90126-9. [DOI] [PubMed] [Google Scholar]
- Miyahara T., Oh-e Y., Takaine E., Kozuka H. Interaction between cadmium and zinc, copper, or lead in relation to the collagen and mineral content of embryonic chick bone in tissue culture. Toxicol Appl Pharmacol. 1983 Jan;67(1):41–48. doi: 10.1016/0041-008x(83)90242-9. [DOI] [PubMed] [Google Scholar]
- Mouw D. R., Wagner J. G., Kalitis K., Vander A. J., Mayor G. H. The effect of parathyroid hormone on the renal accumulation of lead. Environ Res. 1978 Feb;15(1):20–27. doi: 10.1016/0013-9351(78)90074-9. [DOI] [PubMed] [Google Scholar]
- Mundy G. R. Bone resorption and turnover in health and disease. Bone. 1987;8 (Suppl 1):S9–16. [PubMed] [Google Scholar]
- Nathanson J. A., Bloom F. E. Lead-induced inhibition of brain adenyl cyclase. Nature. 1975 May 29;255(5507):419–420. doi: 10.1038/255419a0. [DOI] [PubMed] [Google Scholar]
- Needleman H. L., Gunnoe C., Leviton A., Reed R., Peresie H., Maher C., Barrett P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979 Mar 29;300(13):689–695. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Intracellular calcium homeostasis. Br Med Bull. 1986 Oct;42(4):353–358. doi: 10.1093/oxfordjournals.bmb.a072152. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Norimatsu H., Talmage R. V. Influence of calcitonin on the initial uptake of lead and mercury by bone. Proc Soc Exp Biol Med. 1979 May;161(1):94–98. doi: 10.3181/00379727-161-40498. [DOI] [PubMed] [Google Scholar]
- Oskarsson A., Fowler B. A. Effects of lead inclusion bodies on subcellular distribution of lead in rat kidney: the relationship to mitochondrial function. Exp Mol Pathol. 1985 Dec;43(3):397–408. doi: 10.1016/0014-4800(85)90076-0. [DOI] [PubMed] [Google Scholar]
- Pearl M., Boxt L. M. Radiographic findings in congenital lead poisoning. Radiology. 1980 Jul;136(1):83–84. doi: 10.1148/radiology.136.1.6770416. [DOI] [PubMed] [Google Scholar]
- Peng T. C., Gitelman H. J., Garner S. C. Acute lead-induced increase in serum calcium in the rat without increased secretion of calcitonin. Proc Soc Exp Biol Med. 1979 Jan;160(1):114–117. doi: 10.3181/00379727-160-40400. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Chenu C., Bird A., Mundy G. R., Roodman G. D. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989 Feb;4(1):113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- Pounds J. G. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: a review. Neurotoxicology. 1984 Fall;5(3):295–331. [PubMed] [Google Scholar]
- Pounds J. G., Mittelstaedt R. A. Mobilization of cellular calcium-45 and lead-210: effect of physiological stimuli. Science. 1983 Apr 15;220(4594):308–310. doi: 10.1126/science.6301003. [DOI] [PubMed] [Google Scholar]
- Pounds J. G., Morrison D., Wright R., Casciano D. A., Shaddock J. G. Effect of lead on calcium-mediated cell function in the isolated rat hepatocyte. Toxicol Appl Pharmacol. 1982 May;63(3):402–408. doi: 10.1016/0041-008x(82)90269-1. [DOI] [PubMed] [Google Scholar]
- Pounds J. G., Rosen J. F. Cellular Ca2+ homeostasis and Ca2+-mediated cell processes as critical targets for toxicant action: conceptual and methodological pitfalls. Toxicol Appl Pharmacol. 1988 Jul;94(3):331–341. doi: 10.1016/0041-008x(88)90275-x. [DOI] [PubMed] [Google Scholar]
- Pounds J. G., Rosen J. F. Cellular metabolism of lead: a kinetic analysis in cultured osteoclastic bone cells. Toxicol Appl Pharmacol. 1986 May;83(3):531–545. doi: 10.1016/0041-008x(86)90236-x. [DOI] [PubMed] [Google Scholar]
- Pounds J. G. The role of cell calcium in current approaches to toxicology. Environ Health Perspect. 1990 Mar;84:7–15. doi: 10.1289/ehp.90847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounds J. G., Wright R., Kodell R. L. Cellular metabolism of lead: a kinetic analysis in the isolated rat hepatocyte. Toxicol Appl Pharmacol. 1982 Oct;66(1):88–101. doi: 10.1016/0041-008x(82)90063-1. [DOI] [PubMed] [Google Scholar]
- Pounds J. G., Wright R., Morrison D., Casciano D. A. Effect of lead on calcium homeostasis in the isolated rat hepatocyte. Toxicol Appl Pharmacol. 1982 May;63(3):389–401. doi: 10.1016/0041-008x(82)90268-x. [DOI] [PubMed] [Google Scholar]
- Prentice R. C., Kopp S. J. Cardiotoxicity of lead at various perfusate calcium concentrations: functional and metabolic responses of the perfused rat heart. Toxicol Appl Pharmacol. 1985 Dec;81(3 Pt 1):491–501. doi: 10.1016/0041-008x(85)90420-x. [DOI] [PubMed] [Google Scholar]
- Price P. A. Role of vitamin-K-dependent proteins in bone metabolism. Annu Rev Nutr. 1988;8:565–583. doi: 10.1146/annurev.nu.08.070188.003025. [DOI] [PubMed] [Google Scholar]
- Rabinowitz M. B., Wetherill G. W., Kopple J. D. Kinetic analysis of lead metabolism in healthy humans. J Clin Invest. 1976 Aug;58(2):260–270. doi: 10.1172/JCI108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (1). N Engl J Med. 1986 Apr 24;314(17):1094–1101. doi: 10.1056/NEJM198604243141707. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (2). N Engl J Med. 1986 May 1;314(18):1164–1170. doi: 10.1056/NEJM198605013141807. [DOI] [PubMed] [Google Scholar]
- Reid I. R., Civitelli R., Halstead L. R., Avioli L. V., Hruska K. A. Parathyroid hormone acutely elevates intracellular calcium in osteoblastlike cells. Am J Physiol. 1987 Jul;253(1 Pt 1):E45–E51. doi: 10.1152/ajpendo.1987.253.1.E45. [DOI] [PubMed] [Google Scholar]
- Richardt G., Federolf G., Habermann E. Affinity of heavy metal ions to intracellular Ca2+-binding proteins. Biochem Pharmacol. 1986 Apr 15;35(8):1331–1335. doi: 10.1016/0006-2952(86)90278-9. [DOI] [PubMed] [Google Scholar]
- Rodolfo-Sioson S. A., Ahrens F. A. The effects of lead on collagen biosynthesis in neonatal rats. Res Commun Chem Pathol Pharmacol. 1980 Aug;29(2):317–328. [PubMed] [Google Scholar]
- Rosen J. F., Chesney R. W., Hamstra A., DeLuca H. F., Mahaffey K. R. Reduction in 1,25-dihydroxyvitamin D in children with increased lead absorption. N Engl J Med. 1980 May 15;302(20):1128–1131. doi: 10.1056/NEJM198005153022006. [DOI] [PubMed] [Google Scholar]
- Rosen J. F., Markowitz M. E. D-penicillamine: its actions on lead transport in bone organ culture. Pediatr Res. 1980 Apr;14(4 Pt 1):330–335. doi: 10.1203/00006450-198004000-00014. [DOI] [PubMed] [Google Scholar]
- Rosen J. F., Pounds J. G. Quantitative interactions between Pb2+ and Ca2+ homeostasis in cultured osteoclastic bone cells. Toxicol Appl Pharmacol. 1989 May;98(3):530–543. doi: 10.1016/0041-008x(89)90181-6. [DOI] [PubMed] [Google Scholar]
- Rosen J. F. The metabolism of lead in isolated bone cell populations: interactions between lead and calcium. Toxicol Appl Pharmacol. 1983 Oct;71(1):101–112. doi: 10.1016/0041-008x(83)90049-2. [DOI] [PubMed] [Google Scholar]
- Rosen J. F., Wexler E. E. Studies of lead transport in bone organ culture. Biochem Pharmacol. 1977 Apr 1;26(7):650–652. doi: 10.1016/0006-2952(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Rónai A. Z., Serfózó P., Hársing L. G., Jr, Vizi S. E. The conversion of Met-enkephalin-Arg6, Phe7 to Met-enkephalin in rabbit ear artery. Life Sci. 1983;33 (Suppl 1):101–104. doi: 10.1016/0024-3205(83)90454-x. [DOI] [PubMed] [Google Scholar]
- Saenger P., Markowitz M. E., Rosen J. F. Depressed excretion of 6 beta-hydroxycortisol in lead-toxic children. J Clin Endocrinol Metab. 1984 Feb;58(2):363–367. doi: 10.1210/jcem-58-2-363. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Dowd T. L., Gupta R. K., Rosen J. F. Lead increases free Ca2+ concentration in cultured osteoblastic bone cells: simultaneous detection of intracellular free Pb2+ by 19F NMR. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5133–5135. doi: 10.1073/pnas.86.13.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Angle C., Pitcher H. Relationship between childhood blood lead levels and stature. Pediatrics. 1986 Mar;77(3):281–288. [PubMed] [Google Scholar]
- Siegel G. J., Fogt S. M. Inhibition by lead ion of Electrophorus electroplax (Na+ + K+)-adenosine triphosphatase and K+-p-nitrophenylphosphatase. J Biol Chem. 1977 Aug 10;252(15):5201–5205. [PubMed] [Google Scholar]
- Silbergeld E. K., Schwartz J., Mahaffey K. Lead and osteoporosis: mobilization of lead from bone in postmenopausal women. Environ Res. 1988 Oct;47(1):79–94. doi: 10.1016/s0013-9351(88)80023-9. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Influence of lead ions on cation permeability in human red cell ghosts. J Membr Biol. 1985;84(1):61–71. doi: 10.1007/BF01871648. [DOI] [PubMed] [Google Scholar]
- Simons T. J., Pocock G. Lead enters bovine adrenal medullary cells through calcium channels. J Neurochem. 1987 Feb;48(2):383–389. doi: 10.1111/j.1471-4159.1987.tb04105.x. [DOI] [PubMed] [Google Scholar]
- Slavin R. E., Swedo J., Cartwright J., Jr, Viegas S., Custer E. M. Lead arthritis and lead poisoning following bullet wounds: a clinicopathologic, ultrastructural, and microanalytic study of two cases. Hum Pathol. 1988 Feb;19(2):223–235. doi: 10.1016/s0046-8177(88)80353-8. [DOI] [PubMed] [Google Scholar]
- Smith C. M., DeLuca H. F., Tanaka Y., Mahaffey K. R. Effect of lead ingestion on functions of vitamin D and its metabolites. J Nutr. 1981 Aug;111(8):1321–1329. doi: 10.1093/jn/111.8.1321. [DOI] [PubMed] [Google Scholar]
- Steenhout A. Kinetics of lead storage in teeth and bones: an epidemiologic approach. Arch Environ Health. 1982 Jul-Aug;37(4):224–231. doi: 10.1080/00039896.1982.10667569. [DOI] [PubMed] [Google Scholar]
- Stevenson A. J., Kacew S., Singhal R. L. Influence of lead on hepatic, renal and pulmonary nucleic acid, polyamine, and cyclic adenosine 3':5'-monophosphate metabolism in neonatal rats. Toxicol Appl Pharmacol. 1977 Apr;40(1):161–170. doi: 10.1016/0041-008x(77)90127-2. [DOI] [PubMed] [Google Scholar]
- Stoclet J. C., Gérard D., Kilhoffer M. C., Lugnier C., Miller R., Schaeffer P. Calmodulin and its role in intracellular calcium regulation. Prog Neurobiol. 1987;29(4):321–364. doi: 10.1016/0301-0082(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Talmage R. V., VanderWiel C. J. A study of the action of calcitonin by its effects on lead-induced hypercalcemia. Calcif Tissue Int. 1979;29(3):219–224. doi: 10.1007/BF02408084. [DOI] [PubMed] [Google Scholar]
- Talmage R. V., VanderWiel C. J. Reduction of lead-induced hypercalcemia by calcitonin: comparison between thyroid-intact and thyroidectomized rats. Calcif Tissue Int. 1982 Jan;34(1):97–102. doi: 10.1007/BF02411215. [DOI] [PubMed] [Google Scholar]
- Thomson B. M., Mundy G. R., Chambers T. J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987 Feb 1;138(3):775–779. [PubMed] [Google Scholar]
- Tonge J. I., Burry A. F., Saal J. R. Cerebellar calcification: a possible marker of lead poisoning. Pathology. 1977 Oct;9(4):289–300. doi: 10.3109/00313027709094449. [DOI] [PubMed] [Google Scholar]
- Triffitt J. T. The special proteins of bone tissue. Clin Sci (Lond) 1987 Apr;72(4):399–408. doi: 10.1042/cs0720399. [DOI] [PubMed] [Google Scholar]
- Urist M. R., DeLange R. J., Finerman G. A. Bone cell differentiation and growth factors. Science. 1983 May 13;220(4598):680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Verbeeck R. M., Lassuyt C. J., Heijligers H. J., Driessens F. C., Vrolijk J. W. Lattice parameters and cation distribution of solid solutions of calcium and lead hydroxyapatite. Calcif Tissue Int. 1981;33(3):243–247. doi: 10.1007/BF02409444. [DOI] [PubMed] [Google Scholar]
- Vistica D. T., Ahrens F. A., Ellison W. R. The effects of lead upon collagen synthesis and proline hydroxylation in the Swiss mouse 3T6 fibroblast. Arch Biochem Biophys. 1977 Feb;179(1):15–23. doi: 10.1016/0003-9861(77)90081-9. [DOI] [PubMed] [Google Scholar]
- Warner G. L., Lawrence D. A. The effect of metals on IL-2-related lymphocyte proliferation. Int J Immunopharmacol. 1988;10(5):629–637. doi: 10.1016/0192-0561(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Yamamoto T. Effects of thyrocalcitonin and actinomycin D on the calcium concentration of serum and liver in rats treated with lead. Toxicol Appl Pharmacol. 1974 Aug;29(2):223–228. doi: 10.1016/0041-008x(74)90059-3. [DOI] [PubMed] [Google Scholar]
- de Vellis J., McGinnis J. F., Cole R. Selective effects of lead on the hormonal regulation of glial cell differentiation in cell culture. Prog Clin Biol Res. 1987;253:217–227. [PubMed] [Google Scholar]
- van Leeuwen J. P., Bos M. P., Herrmann-Erlee M. P. Independent and interrelated regulation of ornithine decarboxylase by calcium and cAMP in fetal rat osteoblasts. Cell Calcium. 1988 Aug;9(4):181–191. doi: 10.1016/0143-4160(88)90022-x. [DOI] [PubMed] [Google Scholar]


