Abstract
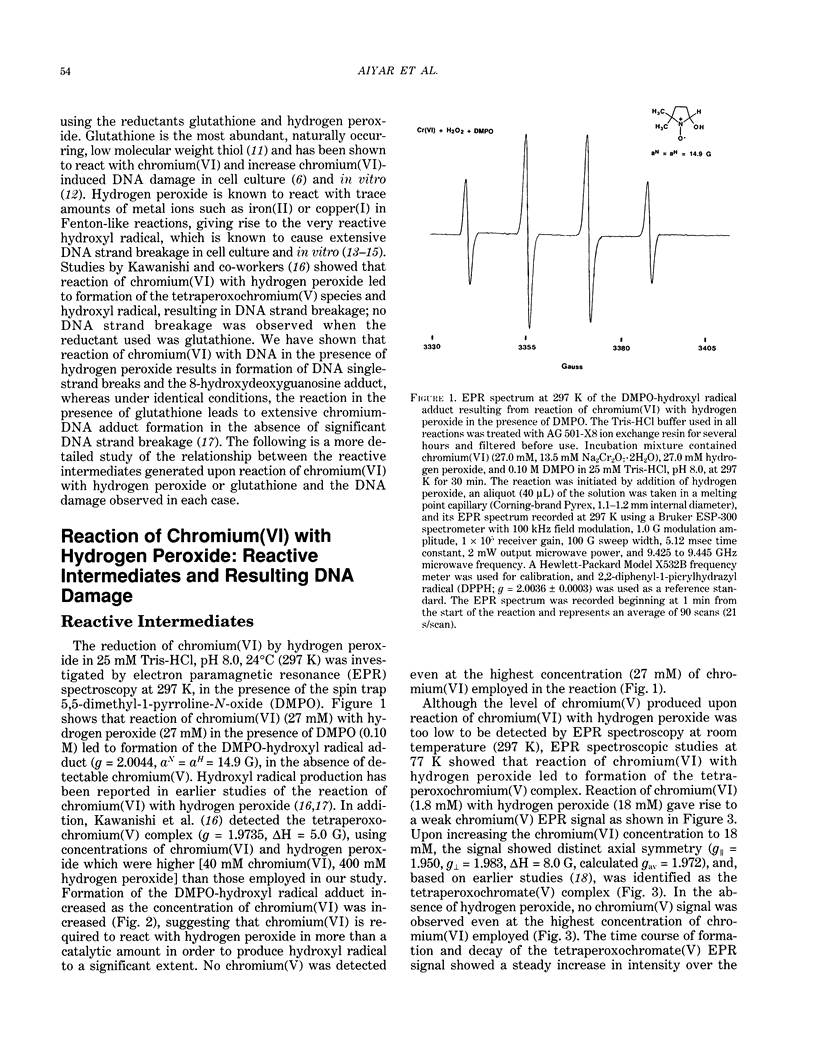
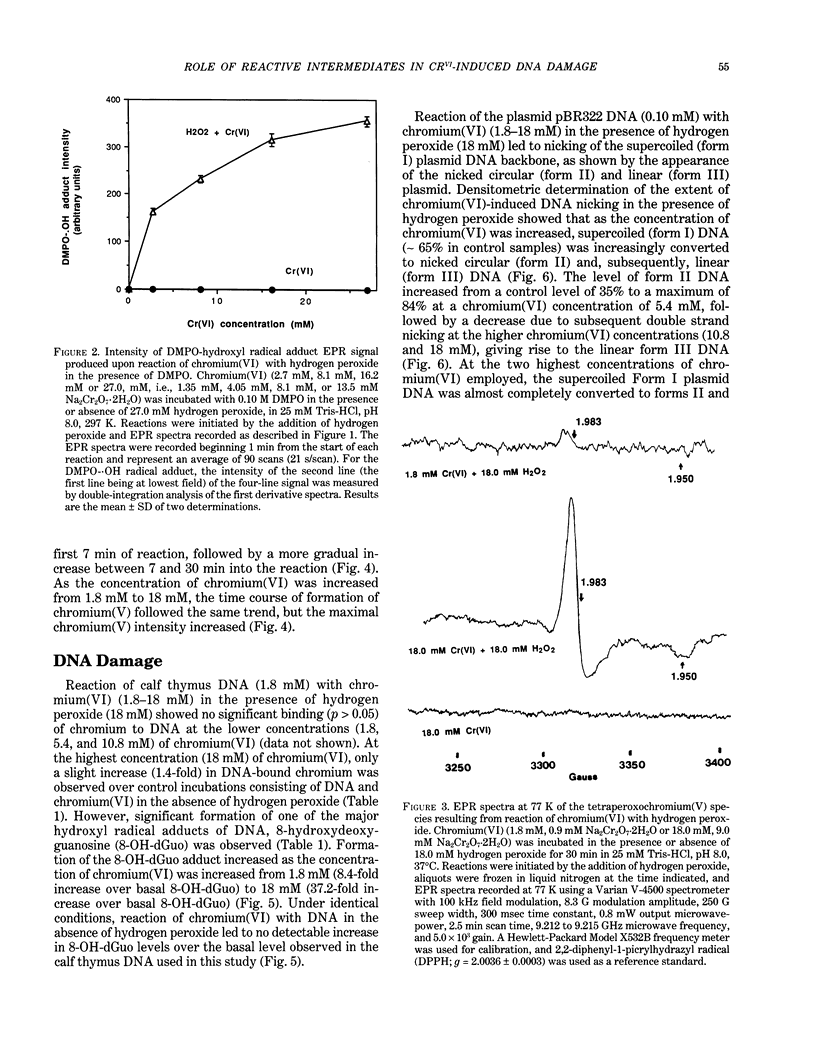
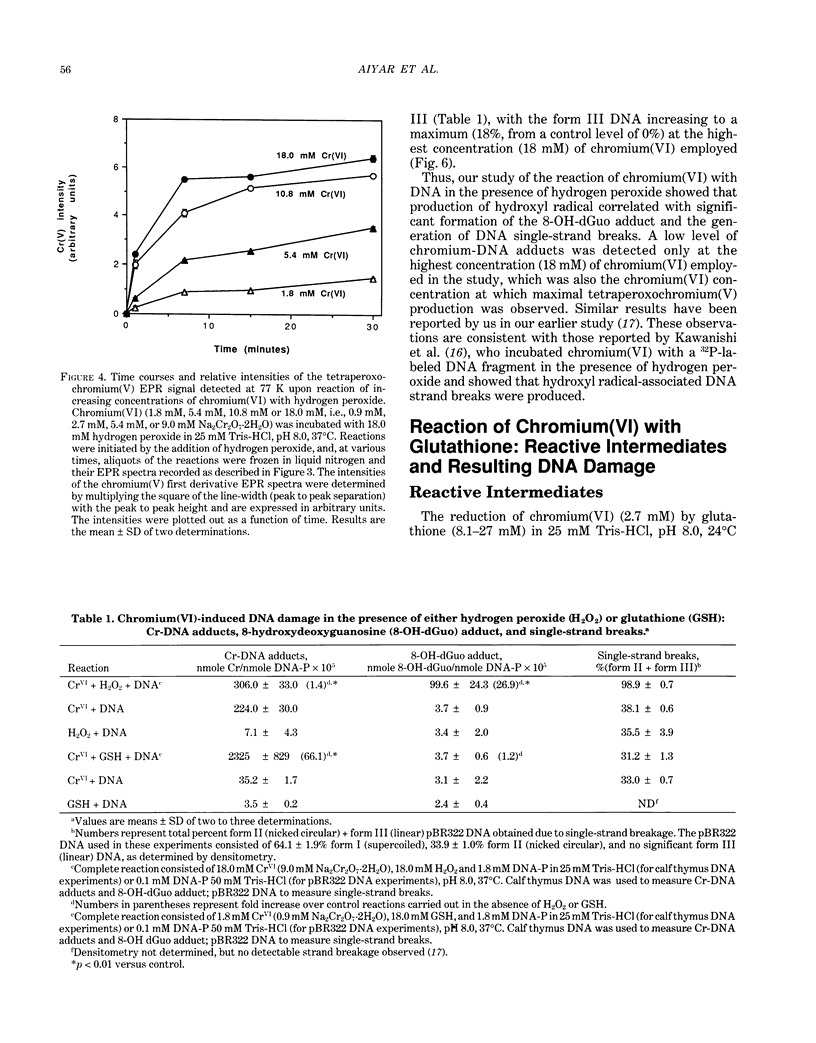
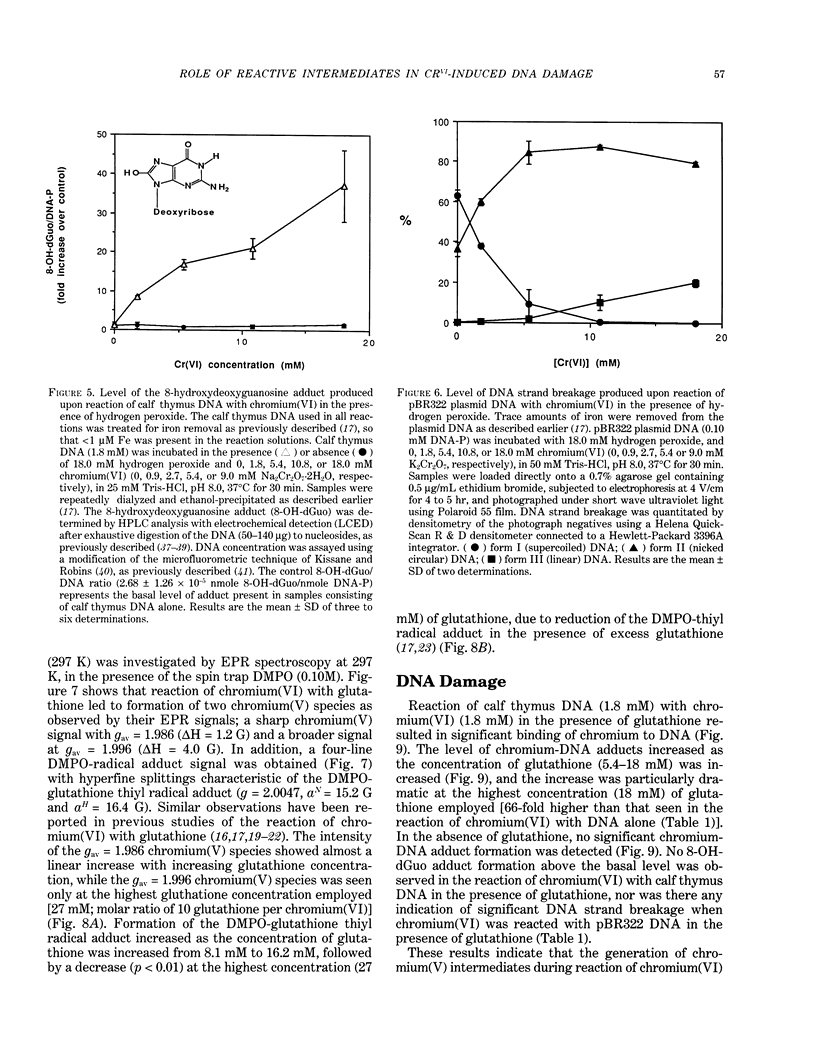
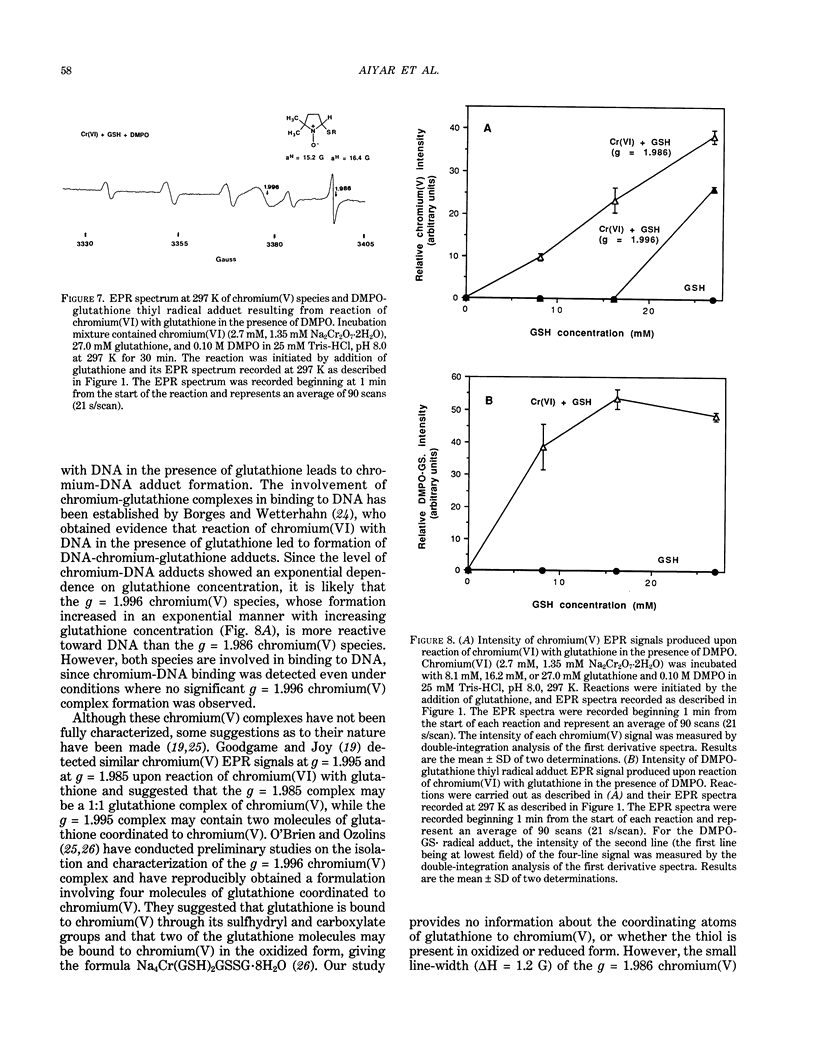
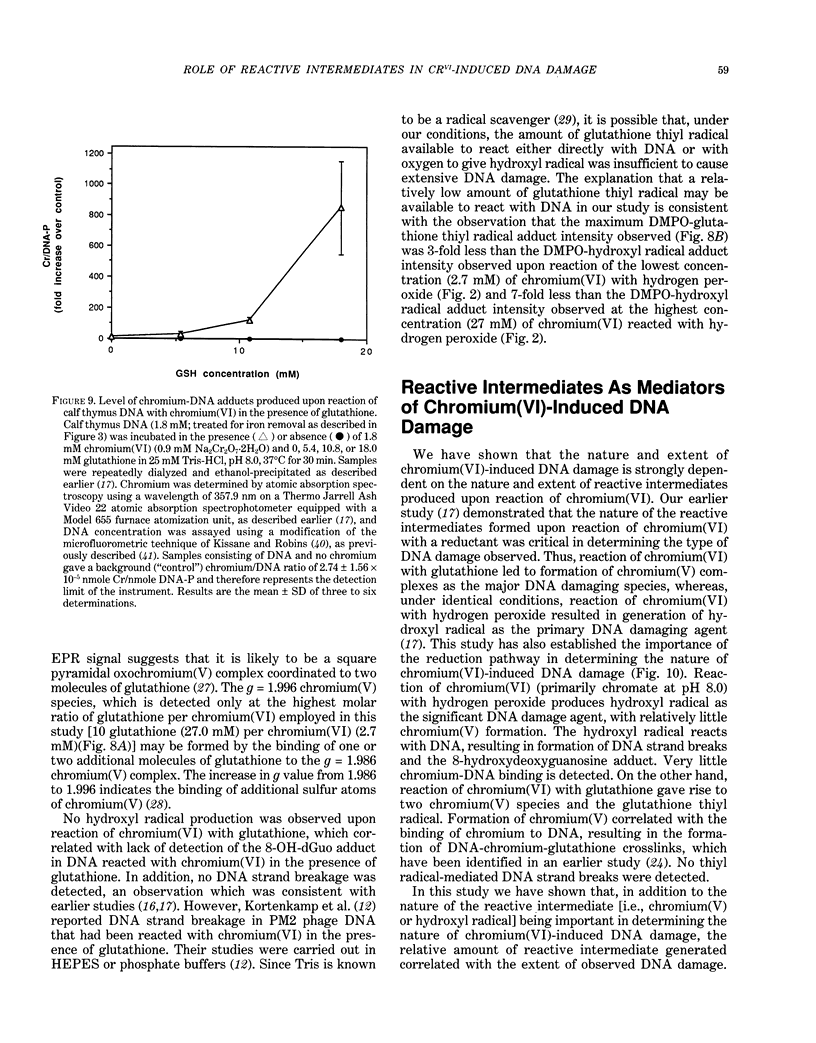
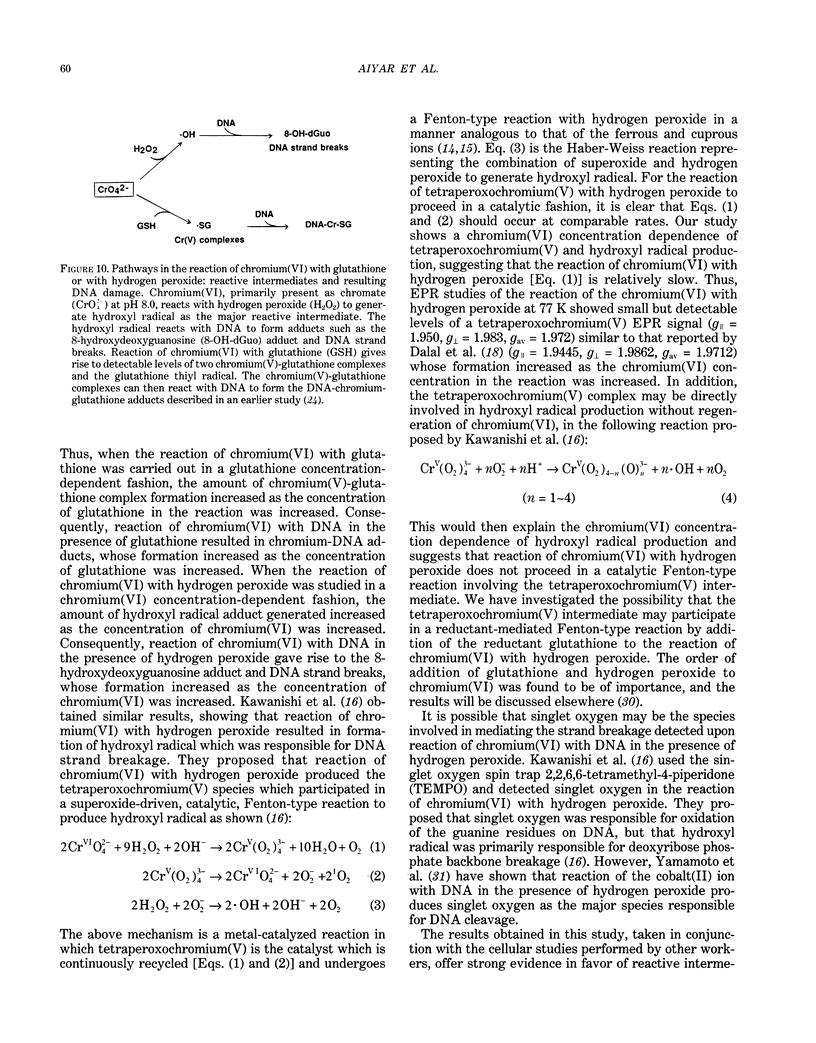
The types of reactive intermediates generated upon reduction of chromium(VI) by glutathione or hydrogen peroxide and the resulting DNA damage have been determined. In vitro, reaction of chromium(VI) with glutathione led to formation of two chromium(V) complexes and the glutathione thiyl radical. When chromium(VI) was reacted with DNA in the presence of glutathione, chromium-DNA adducts were obtained, with no DNA strand breakage. The level of chromium-DNA adduct formation correlated with chromium(V) formation. Reaction of chromium(VI) with hydrogen peroxide led to formation of hydroxyl radical. No chromium(V) was detectable at 24 degrees C (297 K); however, low levels of the tetraperoxochromium(V) complex were detected at 77 K. Reaction of chromium(VI) with DNA in the presence of hydrogen peroxide produced significant DNA strand breakage and the 8-hydroxydeoxyguanosine adduct, whose formation correlated with hydroxyl radical production. No significant chromium-DNA adduct formation was detected. Thus, the nature of chromium(VI)-induced DNA damage appears to be dependent on the reactive intermediates, i.e. chromium(V) or hydroxyl radical, produced during the reduction of chromium(VI).
Full text
PDF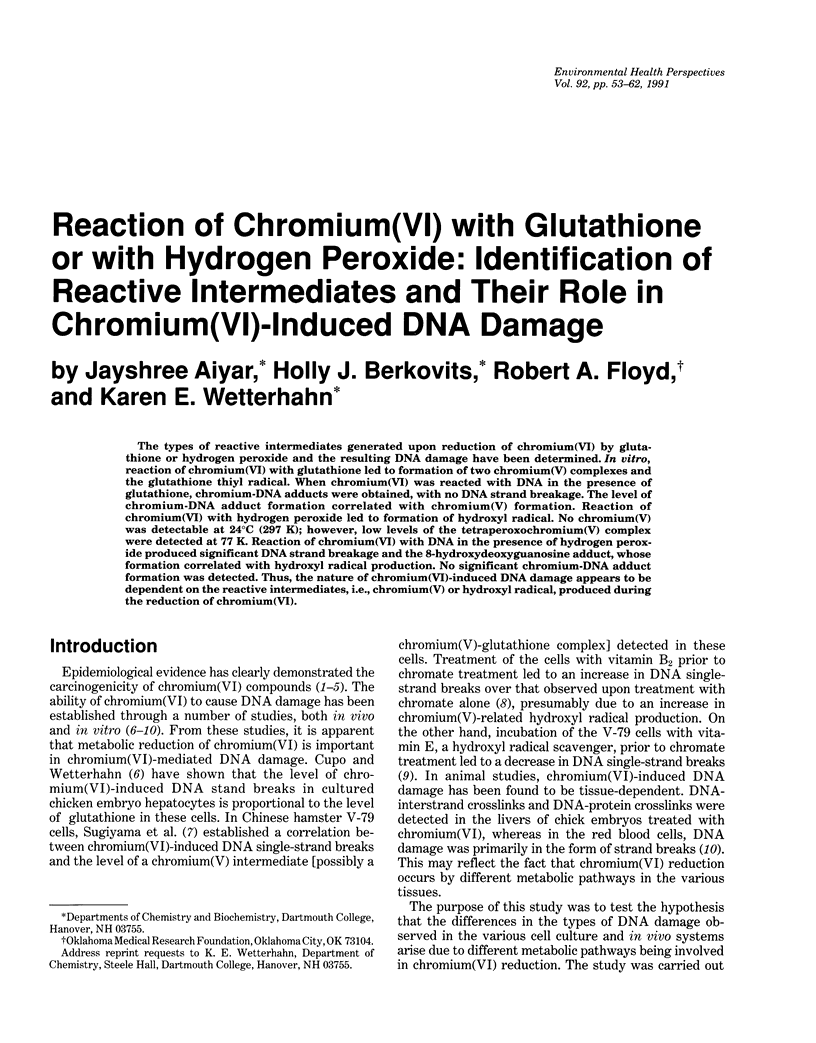


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borges K. M., Wetterhahn K. E. Chromium cross-links glutathione and cysteine to DNA. Carcinogenesis. 1989 Nov;10(11):2165–2168. doi: 10.1093/carcin/10.11.2165. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R. B., Wetterhahn K. E. Nickel distribution and DNA lesions induced in rat tissues by the carcinogen nickel carbonate. Cancer Res. 1982 Sep;42(9):3544–3549. [PubMed] [Google Scholar]
- Cupo D. Y., Wetterhahn K. E. Modification of chromium(VI)-induced DNA damage by glutathione and cytochromes P-450 in chicken embryo hepatocytes. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6755–6759. doi: 10.1073/pnas.82.20.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Harris J., West M., Wong P. K. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986 Jun 13;137(2):841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., West M. S., Eneff K. L., Hogsett W. E., Tingey D. T. Hydroxyl free radical mediated formation of 8-hydroxyguanine in isolated DNA. Arch Biochem Biophys. 1988 Apr;262(1):266–272. doi: 10.1016/0003-9861(88)90188-9. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr Detection of DNA single-strand breaks produced during the repair of damage by DNA-protein cross-linking agents. Cancer Res. 1982 Jan;42(1):145–149. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Seres D. S., Lechner J. F., Harris C. C. DNA-protein cross-linking by chromium salts. Chem Biol Interact. 1981 Sep;36(3):345–354. doi: 10.1016/0009-2797(81)90077-6. [DOI] [PubMed] [Google Scholar]
- Goodgame D. M., Joy A. M. Relatively long-lived chromium(V) species are produced by the action of glutathione on carcinogenic chromium(VI). J Inorg Biochem. 1986 Mar;26(3):219–224. doi: 10.1016/0162-0134(86)80044-7. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Wetterhahn K. E. Chromium (VI)-induced DNA damage in chick embryo liver and blood cells in vivo. Carcinogenesis. 1986 Dec;7(12):2085–2088. doi: 10.1093/carcin/7.12.2085. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Sano S. Mechanism of DNA cleavage induced by sodium chromate(VI) in the presence of hydrogen peroxide. J Biol Chem. 1986 May 5;261(13):5952–5958. [PubMed] [Google Scholar]
- Kim S., Iwai Y., Fujino M., Furumoto M., Sumino K., Miyasaki K. Chromium-induced pulmonary cancer. Report of a case and a review of the literature. Acta Pathol Jpn. 1985 May;35(3):643–654. doi: 10.1111/j.1440-1827.1985.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A., Ozolins Z., Beyersmann D., O'Brien P. Generation of PM2 DNA breaks in the course of reduction of chromium(VI) by glutathione. Mutat Res. 1989 Feb;216(1):19–26. doi: 10.1016/0165-1161(89)90019-8. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Levy L. S., Martin P. A., Bidstrup P. L. Investigation of the potential carcinogenicity of a range of chromium containing materials on rat lung. Br J Ind Med. 1986 Apr;43(4):243–256. doi: 10.1136/oem.43.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Miller C. A., 3rd, Costa M. Characterization of DNA-protein complexes induced in intact cells by the carcinogen chromate. Mol Carcinog. 1988;1(2):125–133. doi: 10.1002/mc.2940010208. [DOI] [PubMed] [Google Scholar]
- Paschen W., Weser U. Problems concerning the biochemical action of superoxide dismutase (erythrocuprein). Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):727–737. doi: 10.1515/bchm2.1975.356.s1.727. [DOI] [PubMed] [Google Scholar]
- Sagripanti J. L., Kraemer K. H. Site-specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen peroxide. J Biol Chem. 1989 Jan 25;264(3):1729–1734. [PubMed] [Google Scholar]
- Schimmack W., Summer K. H. Reaction of the nitroxyl radical TAN with glutathione. Int J Radiat Biol Relat Stud Phys Chem Med. 1978 Sep;34(3):293–296. doi: 10.1080/09553007814550901. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. On the mechanism of the chromate reduction by glutathione: ESR evidence for the glutathionyl radical and an isolable Cr(V) intermediate. Biochem Biophys Res Commun. 1988 Oct 14;156(1):137–142. doi: 10.1016/s0006-291x(88)80815-5. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Ando A., Nakao K., Ueta H., Hidaka T., Ogura R. Influence of vitamin B2 on formation of chromium(V), alkali-labile sites, and lethality of sodium chromate(VI) in Chinese hamster V-79 cells. Cancer Res. 1989 Nov 15;49(22):6180–6184. [PubMed] [Google Scholar]
- Sugiyama M., Ando A., Ogura R. Vitamin B2-enhancement of sodium chromate (VI)--Induced DNA single strand breaks: ESR study of the action of vitamin B2. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1080–1085. doi: 10.1016/0006-291x(89)92219-5. [DOI] [PubMed] [Google Scholar]
- Wedrychowski A., Ward W. S., Schmidt W. N., Hnilica L. S. Chromium-induced cross-linking of nuclear proteins and DNA. J Biol Chem. 1985 Jun 10;260(11):7150–7155. [PubMed] [Google Scholar]
- Yamamoto K., Inoue S., Yamazaki A., Yoshinaga T., Kawanishi S. Site-specific DNA damage induced by cobalt(II) ion and hydrogen peroxide: role of singlet oxygen. Chem Res Toxicol. 1989 Jul-Aug;2(4):234–239. doi: 10.1021/tx00010a004. [DOI] [PubMed] [Google Scholar]


