Abstract
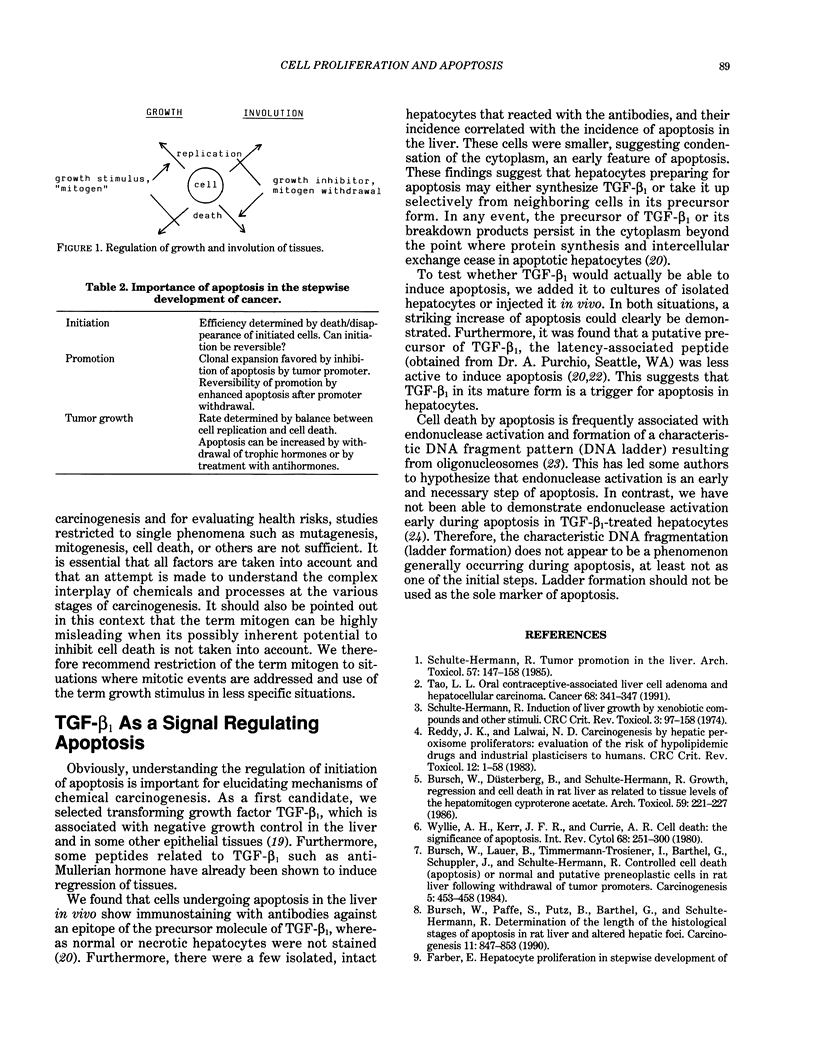
The growth rate of tissues including tumors is determined by the difference between cell replication and cell death. Among different types of cell death, apoptosis, a form of programmed cell death, is of particular importance. Nongenotoxic carcinogens exert their carcinogenic effects not only via stimulation of cell replication but also by modulating the incidence of apoptosis. This can be seen at different stages of carcinogenesis: a) After initiation in the liver, many initiated cells may undergo apoptosis and never develop into preneoplastic foci, as suggested by both biological and mathematical studies. Thus, apoptosis appears to determine the efficiency of initiation. b) In the promotion stage, early preneoplastic hepatic foci originate either from treatment with a genotoxic carcinogen or spontaneously exhibit much higher rates of cell replication than normal cells, but nevertheless show little preferential growth. This is due to enhanced rates of apoptosis. Some tumor promoters were found to inhibit apoptosis and thereby accelerate foci growth and carcinogenesis. c) In neoplastic nodules and tumors, apoptosis has been shown to be an important growth determinant and to be regulated by growth regulatory hormones, which thereby may decrease or accelerate tumor growth. Studies on the regulation of apoptosis revealed that in the liver, transforming growth factor TGF-beta 1 is involved in the initiation of apoptosis. This was based on three lines of evidence: TGF-beta 1 induced apoptosis in isolated hepatocytes, b) in vivo hepatocytes undergoing apoptosis showed positive immunostaining with antibodies against a precursor of TGF-beta 1.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bursch W., Düsterberg B., Schulte-Hermann R. Growth, regression and cell death in rat liver as related to tissue levels of the hepatomitogen cyproterone acetate. Arch Toxicol. 1986 Dec;59(4):221–227. doi: 10.1007/BF00290542. [DOI] [PubMed] [Google Scholar]
- Bursch W., Lauer B., Timmermann-Trosiener I., Barthel G., Schuppler J., Schulte-Hermann R. Controlled death (apoptosis) of normal and putative preneoplastic cells in rat liver following withdrawal of tumor promoters. Carcinogenesis. 1984 Apr;5(4):453–458. doi: 10.1093/carcin/5.4.453. [DOI] [PubMed] [Google Scholar]
- Bursch W., Liehr J. G., Sirbasku D. A., Putz B., Taper H., Schulte-Hermann R. Control of cell death (apoptosis) by diethylstilbestrol in an estrogen-dependent kidney tumor. Carcinogenesis. 1991 May;12(5):855–860. doi: 10.1093/carcin/12.5.855. [DOI] [PubMed] [Google Scholar]
- Bursch W., Paffe S., Putz B., Barthel G., Schulte-Hermann R. Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis. 1990 May;11(5):847–853. doi: 10.1093/carcin/11.5.847. [DOI] [PubMed] [Google Scholar]
- Carr B. I., Hayashi I., Branum E. L., Moses H. L. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986 May;46(5):2330–2334. [PubMed] [Google Scholar]
- Columbano A., Ledda-Columbano G. M., Ennas M. G., Curto M., Chelo A., Pani P. Cell proliferation and promotion of rat liver carcinogenesis: different effect of hepatic regeneration and mitogen induced hyperplasia on the development of enzyme-altered foci. Carcinogenesis. 1990 May;11(5):771–776. doi: 10.1093/carcin/11.5.771. [DOI] [PubMed] [Google Scholar]
- Farber E. Hepatocyte proliferation in stepwise development of experimental liver cell cancer. Dig Dis Sci. 1991 Jul;36(7):973–978. doi: 10.1007/BF01297150. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5(5):545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Isaacs J. T. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990 Jun 15;50(12):3748–3753. [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor thirty-five years later. In Vitro Cell Dev Biol. 1987 Apr;23(4):227–238. doi: 10.1007/BF02623703. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Jondal M., Orrenius S. Inhibition of DNA fragmentation in thymocytes and isolated thymocyte nuclei by agents that stimulate protein kinase C. J Biol Chem. 1989 Aug 15;264(23):13399–13402. [PubMed] [Google Scholar]
- Moolgavkar S. H., Luebeck E. G., de Gunst M., Port R. E., Schwarz M. Quantitative analysis of enzyme-altered foci in rat hepatocarcinogenesis experiments--I. Single agent regimen. Carcinogenesis. 1990 Aug;11(8):1271–1278. doi: 10.1093/carcin/11.8.1271. [DOI] [PubMed] [Google Scholar]
- Oberhammer F. A., Pavelka M., Sharma S., Tiefenbacher R., Purchio A. F., Bursch W., Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F., Bursch W., Parzefall W., Breit P., Erber E., Stadler M., Schulte-Hermann R. Effect of transforming growth factor beta on cell death of cultured rat hepatocytes. Cancer Res. 1991 May 1;51(9):2478–2485. [PubMed] [Google Scholar]
- Pitot H. C., Sirica A. E. The stages of initiation and promotion in hepatocarcinogenesis. Biochim Biophys Acta. 1980 May 6;605(2):191–215. doi: 10.1016/0304-419x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Rabes H. M., Bücher T., Hartmann A., Linke I., Dünnwald M. Clonal growth of carcinogen-induced enzyme-deficient preneoplastic cell populations in mouse liver. Cancer Res. 1982 Aug;42(8):3220–3227. [PubMed] [Google Scholar]
- Reddy J. K., Lalwai N. D. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit Rev Toxicol. 1983;12(1):1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- Satoh K., Hatayama I., Tateoka N., Tamai K., Shimizu T., Tatematsu M., Ito N., Sato K. Transient induction of single GST-P positive hepatocytes by DEN. Carcinogenesis. 1989 Nov;10(11):2107–2111. doi: 10.1093/carcin/10.11.2107. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R. Induction of liver growth by xenobiotic compounds and other stimuli. CRC Crit Rev Toxicol. 1974 Sep;3(1):97–158. doi: 10.3109/10408447409079856. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R., Timmermann-Trosiener I., Barthel G., Bursch W. DNA synthesis, apoptosis, and phenotypic expression as determinants of growth of altered foci in rat liver during phenobarbital promotion. Cancer Res. 1990 Aug 15;50(16):5127–5135. [PubMed] [Google Scholar]
- Schulte-Hermann R. Tumor promotion in the liver. Arch Toxicol. 1985 Aug;57(3):147–158. doi: 10.1007/BF00290879. [DOI] [PubMed] [Google Scholar]
- Tao L. C. Oral contraceptive-associated liver cell adenoma and hepatocellular carcinoma. Cytomorphology and mechanism of malignant transformation. Cancer. 1991 Jul 15;68(2):341–347. doi: 10.1002/1097-0142(19910715)68:2<341::aid-cncr2820680223>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tatematsu M., Mera Y., Ito N., Satoh K., Sato K. Relative merits of immunohistochemical demonstrations of placental, A, B and C forms of glutathione S-transferase and histochemical demonstration of gamma-glutamyl transferase as markers of altered foci during liver carcinogenesis in rats. Carcinogenesis. 1985 Nov;6(11):1621–1626. doi: 10.1093/carcin/6.11.1621. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]


