Abstract
Secretion of proteins by Helicobacter pylori may contribute to gastric inflammation and epithelial damage. An in vitro analysis was designed to identify proteins released by mechanisms other than nonspecific lysis. The radioactivity of proteins in the supernatant was compared with that of the intact organism by two-dimensional gel phosphorimaging following a 4-h pulse-chase. The ratio of the amount of UreB, a known cytoplasmic protein, in the supernatant to that in the pellet was found to be 0.25, and this was taken as an index of lysis during the experiments (n = 6). Ratios greater than that of UreB were used to distinguish proteins that were selectively released into the medium. Thus, proteins enriched more than 10-fold in the supernatant compared to UreB were identified by mass spectrometry. Sixteen such proteins were present in the supernatant: VacA; a conserved secreted protein (HP1286); putative peptidyl cis-trans isomerase (HP0175); six proteins encoded by HP0305, HP0231, HP0973, HP0721, HP0129, and HP0902; thioredoxin (HP1458); single-stranded-DNA-binding 12RNP2 precursor (HP0827); histone-like DNA-binding protein HU (HP0835); ribosomal protein L11 (HP1202); a putative outer membrane protein (HP1564); and outer membrane proteins Omp21 (HP0913) and Omp20 (HP0912). All except HP0902, thioredoxin, HP0827, HP0835, and HP1202 had a signal peptide. When nalidixic acid, a DNA synthesis inhibitor, was added to inhibit cell division but not protein synthesis, to decrease possible contamination due to outer membrane shedding, two outer membrane proteins (Omp21 and Omp20) disappeared from the supernatant, and the amount of VacA also decreased. Thus, 13 proteins were still enriched greater than 10-fold in the medium after nalidixic acid treatment, suggesting these were released specifically, possibly by secretion. These proteins may be implicated in H. pylori-induced effects on the gastric epithelium.
Helicobacter pylori is a noninvasive, gram-negative bacterium that colonizes the gastric mucosa (9, 24, 26). Gastric colonization by H. pylori results in a mucosal inflammatory response and is a risk factor for peptic ulcer disease and gastric malignancy (15, 21, 27, 31). Elucidation of the mechanism underlying inflammation and damage of the underlying tissue is important to understanding the development and progression of these gastrointestinal disorders.
Epithelial cell damage may occur as a direct effect of bacterial habitation or as a consequence of the chronic and acute inflammatory responses induced by H. pylori, which may be due in part to responses to proteins released or injected by the organism (5, 6, 16, 28). Several potential bacterial virulence factors that may contribute to mucosal inflammation and epithelial cell damage have been identified (5, 28). Two of these factors, which are known to differ among H. pylori strains, are a high-molecular-mass protein encoded by cagA (11, 48) and the 87-kDa vacuolating toxin encoded by vacA (13). The latter is toxic to epithelial cells in vitro (13, 14, 29). Although CagA and VacA, both secreted proteins, have been postulated to be major virulence factors in H. pylori, there is no association between CagA or VacA status and clinical outcome in the Oriental population, since, for example, the H. pylori strains found in asymptomatic Japanese and Koreans express both CagA and VacA with the same frequency as the strains found in patients with peptic ulcer or gastric cancer (39, 52).
Gastric infection by H. pylori induces mucosal production of several cytokines in the host, including interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (17-19, 34). IL-8, a potent T-cell and neutrophil recruitment factor, is produced by various cell types, including macrophages, T cells, endothelial cells, and epithelial cells (36), and elevated levels of IL-8 have been reported in a number of inflammatory conditions (46). H. pylori provokes transcription factor NF-κB expression, resulting in IL-8 secretion in host cells (32). Both IL-8 secretion and NF-κB activation have been suggested to be dependent on the expression of proteins encoded by the cag pathogenicity island. In addition, the finding that most of IL-8 secretion and NF-κB activation disappeared in strains with mutations in picB, also called cagE, a recently identified gene that encodes part of the type IV secretion apparatus of cag (32), suggests that at least some cag-encoded proteins may be secreted. These pleiotropic responses suggest that several pathogenic proteins secreted or released by H. pylori might produce inflammation and consequent damage to the gastric epithelium.
It has been difficult to identify the proteins secreted by H. pylori because of the high frequency of lysis of H. pylori, which results in nonspecific release of all cytoplasmic proteins (8, 43, 49). In order to minimize contamination due to lysis, a Ficoll step gradient method was used for separation of the proteins specifically released into the medium from intact bacteria. A pulse-labeling protocol followed by phosphorimaging was then used to further distinguish the proteins secreted or specifically released into the medium during the 4-h labeling period. Nalidixic acid was used with the assumption that its blockade of DNA synthesis could further reduce contamination due to membrane shedding during cell division. This compound inhibits DNA synthesis without inhibiting protein synthesis, and this arrests cell division and elaboration of outer and inner membranes.
Thirteen proteins remained that were selectively enriched in the medium containing this inhibitor and thus were not released by membrane shedding.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
Initially, H. pylori (ATCC 43504, type strain 11637, cag+ vacA+) was grown in brain heart infusion medium (Difco, Le Pont de Claix, France) containing 5% horse serum (Gibco, Grand island, N.Y.) for 2 to 3 days at 37°C in 10% CO2 and 5% O2 until it reached an optical density at 600 nm of 0.6 to 0.7 (equivalent to 3.3 × 108 to 3.8 × 108 CFU/ml), corresponding to the mid-exponential growth phase. After washing by centrifugation (10 min, 1,500 × g, room temperature) with RPMI-based methionine-free minimal medium 1640 (Gibco, Grand Island, N.Y.), H. pylori was grown in RPMI-based methionine-free minimal medium 1640 supplemented with 0.4% β-cyclodextrin (Sigma, St. Louis, Mo.).
Protein labeling and separation.
A pulse-chase protocol was employed to distinguish newly synthesized and secreted proteins from contaminating proteins released by cell lysis. After 16 h, when the mean optical density at 600 nm was 0.86, 100 μCi of [35S]methionine (ICN, Irvine, Calif.) was added, the bacteria were incubated for another 4 h in medium containing unlabeled methionine, and the mean optical density at 600 nm increased to 0.94, showing that growth continued under these conditions.
The intact bacteria were then separated from the medium by centrifugation (10 min, 10,000 × g, 4°C) through a 10% Ficoll (Sigma, St. Louis, Mo.) cushion containing 0.25 M sucrose so as to minimize contamination due to cell rupture. The cell-free supernatant was centrifuged again through 10% Ficoll containing 0.25 M sucrose to remove any cellular debris (1 h, 100,000 × g, 4°C). The protein in the supernatant was concentrated by centrifugation with nominal 3,000-molecular-weight-cutoff Centriprep YM-3 tubes (Millipore, Bedford, Mass.) as described by the manufacturer. When the initial 90 ml of supernatant which was aspirated from the top of the Ficoll layer was concentrated to 1 to 1.5 ml, the protein concentration was around 1 to 3 μg/μl by the method of Lowry et al. (29a). After a volume containing 800 μg of protein was taken, this supernatant was concentrated further by Speedvac treatment.
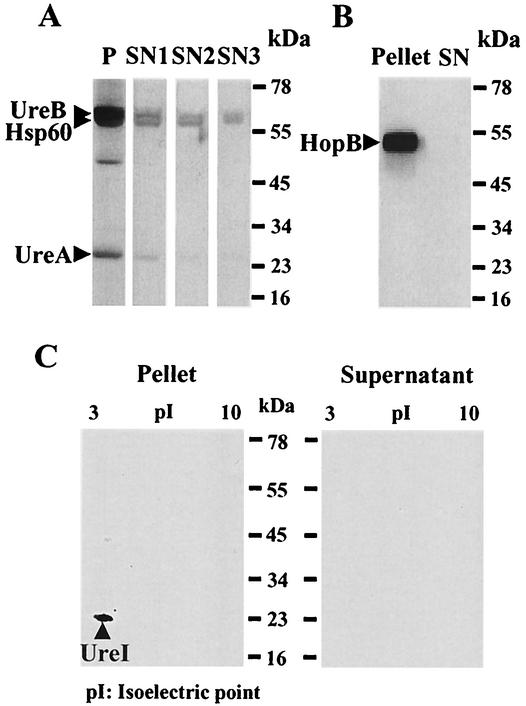
The two-step centrifugation method on a 10% Ficoll step gradient was selected after comparing three different centrifugation protocols. First, the supernatant was separated from bacteria by centrifugation at 1,500 × g for 10 min without Ficoll. Second, a one-step 100,000 × g centrifugation over 10% Ficoll for 1 h was analyzed, and finally we employed a two-stage centrifugation consisting of 10,000 × g on 10% Ficoll for 10 min and 100,000 × g on 10% Ficoll for 1 h. We compared the amounts of UreA, UreB, and Hsp60, known cytoplasmic proteins, in the pellet and in the supernatant. HopB, an outer membrane protein (22), was used as a qualitative indicator of outer membrane protein breakage, and UreI, an inner membrane protein (50), was used as a qualitative indicator of inner membrane breakage.
Western analysis of the supernatant and pellet was performed with anti-UreA (1:400,000 dilution), anti-UreB (1:150,000 dilution) (both gifts from H. L. T. Mobley, University of Maryland), and anti-Hsp60 polyclonal antibody (1:1,500,000 dilution) (Stress Gen Biotechnologies Corp., Victoria, Canada). To compare the amounts of HopB and UreI in the pellet and in the supernatant, Western analysis was done with an anti-HopB polyclonal antibody (1:50,000 dilution) (gift from P. Doig and T. J. Trust, University of Victoria, Canada) and with anti-UreI polyclonal antibody (1:2,000 dilution) (Alpha Diagnostics, San Antonio, Tex.).
The supernatant that was separated from intact bacteria by a single centrifugation step of 1,500 × g for 10 min without a step gradient showed evident staining for UreB (61.1 kDa) and Hsp60 (58.3 kDa) and faint staining for UreA (26.5 kDa) but much less than in the pellet (Fig. 1A). These bands were much decreased in the supernatant with centrifugation (100,000 × g for 1 h) through 10% Ficoll. The intensities of the UreB and Hsp60 bands were further decreased and the UreA band was almost invisible in the supernatant when a second centrifugation step (1 h, 100,000 × g) was used to remove membrane fragments from the supernatant of the Ficoll step separation.
FIG. 1.
(A) Western analysis of UreA, UreB, and Hsp60 obtained by three different centrifugation methods. The pellet (P) showed clear bands of UreB (61.1 kDa), Hsp60 (58.3 kDa), and UreA (26.5 kDa), which were still strong in the supernatant (SN1) after 1,500 × g centrifugation without Ficoll for 10 min. These bands subsequently decreased in the supernatant (SN2) after 100,000 × g centrifugation with Ficoll for 1 h and became fainter in the supernatant (SN3) after two-step centrifugation (10,000 × g for 10 min and 100,000 × g for 1 h). (B) Western analysis of HopB. HopB (56.8 kDa), an outer membrane protein, showed intense staining in the H. pylori pellet but was not visible in the supernatant (SN). (C) Western analysis of UreI following two-dimensional gel electrophoresis of pellet and supernatant. UreI (21.7 kDa; pI, 5.81), an inner membrane protein, was seen in the pellet but not in the supernatant of H. pylori.
With this two-step centrifugation method, HopB (56.8 kDa) showed intense staining in the H. pylori pellet but was not visible in the supernatant (Fig. 1B). UreI (21.7 kDa; isoelectric point, 5.81), which was present in the two-dimensional gel of the H. pylori pellet, was also not visible in the two-dimensional gel of the supernatant (Fig. 1C), indicating that there was minimal contamination by either outer or inner membrane proteins with this procedure. This two-step centrifugation method was then used to identify proteins enriched in the supernatant. H. pylori can divide during the 4-h incubation period used for protein labeling. This involves elaboration of outer membrane structure and possible contamination of the medium with outer membrane proteins or proteins embedded in the outer membrane in transit. Cell division was reduced by preincubation of H. pylori with 100 μg of nalidixic acid (Sigma, St. Louis, Mo.), a DNA synthesis inhibitor (20), per ml for 1 h before adding [35S]methionine.
Two-dimensional gel electrophoresis and mass spectrometry.
Bacterial pellet protein (800 μg) and the concentrated supernatant were separated by two-dimensional gel electrophoresis (isoelectric focusing followed by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]). Isoelectric focusing was carried out on Immobiline Dry strips (pH 3 to 10, 11 cm) (Pharmacia Biotech AB, Uppsala, Sweden) for 30850 V·h (Multiphor II electrophoretic system; Pharmacia Biotech AB, Uppsala, Sweden). The strips were then sealed to the top of the stacking gel, which was placed on the top of a 5 to 21% gradient SDS-acrylamide slab gel. After running the gel and drying by vacuum, it was analyzed by phosphorimaging (Molecular Dynamics, Sunnyvale, Calif.). The intensity of each protein spot was measured by densitometry. Proteins with high radioactivity in the supernatant compared to intact bacteria were selected for mass spectrometry on the assumption that these were secreted or released specifically rather than simply via lysis of the intact organism. After finding out the identities of enhanced proteins in the supernatant, the nature of some of the hypothetical proteins was found by homology with the Blast search program.
RESULTS
Supernatant proteins.
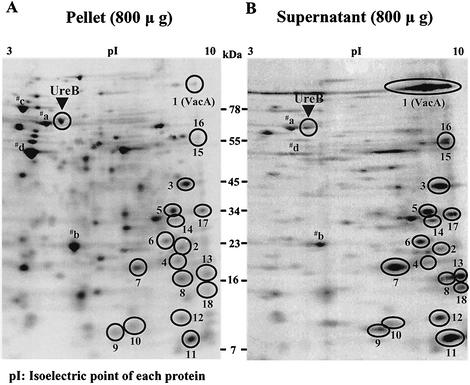
When labeling of the cytoplasmic protein UreB (30) in the supernatant was compared with that in the pellet with the Scion program, the mean ratio of radioactivity was 0.25 (n = 6). This ratio was similar to that of four other prominent cytoplasmic proteins, which were identified by mass spectrometry as Hsp60 (spot a), nonheme iron-containing ferritin (spot b), Hsp70 (spot c), and elongation factor TU (spot d) (Fig. 2). The average ratio of these five cytoplasmic proteins in the supernatant compared to the pellet was approximately that of UreB. Furthermore, the ratio of most proteins analyzed was similar to that of UreB (Fig. 2); hence, these are neither secreted nor selectively released but appear in the supernatant due to lysis of the bacteria.
FIG. 2.
Phosphorimages following two-dimensional gel electrophoresis of H. pylori in the pellet (A) and supernatant (B) after [35S]methionine pulse-labeling with the two-step centrifugation method. When phosphorimages of labeled proteins were compared, 18 spots were enriched in the supernatant compared to the pellet. Spots: 1, HP0887 (VacA); 2, HP1286; 3, HP0175; 4, HP0305; 5, HP0231; 6, HP0973; 7, HP0721; 8, HP0129; 9, HP0902; 10, HP1458; 11, HP0827; 12, HP0835; 13, HP1202; 14, HP1564; 15, HP0913; 16, HP0912; 17, HP1201; 18, HP0720; a, Hsp60; b, nonheme iron-containing ferritin; c, Hsp70; d, elongation factor TU.
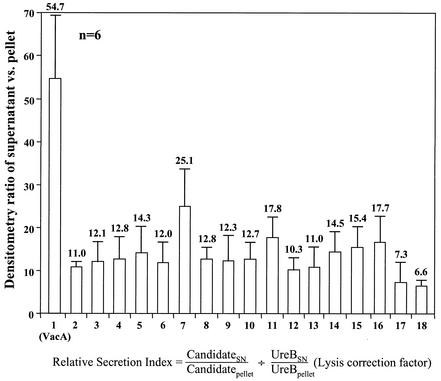
Of the more than 160 spots in the pellet, only 18 were increased in radioactivity in the supernatant. One of these 18 was VacA, identified by Western blot with anti-VacA protein monoclonal antibody (Austral Biologicals, San Ramon, Calif., 1:10,000 dilution). When the radioactivity of these 18 proteins was normalized to UreB radioactivity with the formula [(can-didatesupernatant)/(candidatepellet) divided by (UreBsupernatant)/(UreBpellet), the lysis correction factor in each gel], 16 proteins showed a 10-fold-higher enrichment ratio compared to UreB (Fig. 3). As several enhanced proteins were smaller than 20 kDa, an additional small cytoplasmic marker, nonheme iron-containing ferritin (19.3 kDa, spot b in Fig. 2), was used to rule out nonspecific leakage of these small proteins. The mean ratio of nonheme iron-containing ferritin in the supernatant over the pellet was 0.31, and these 16 proteins again showed a 10-fold-higher enrichment ratio compared to this small protein marker. This 10-fold ratio was taken as indicative of specific release by the organism. Interestingly, all 16 of these putative secreted proteins were found to have a fairly high isoelectric point (pI) in the alkaline range (Fig. 2). In addition, there were another two spots but only with a 7.3- and 6.6-fold enrichment (Fig. 3). All 18 proteins were identified by mass spectrometry.
FIG. 3.
Densitometry ratio of 18 proteins in the supernatant versus the pellet in the 35S-labeled phosphorimages of two-dimensional gel electrophoresis. When these proteins were normalized to UreB, 16 proteins were enriched more than 10-fold in the supernatant compared to the pellet. Data are based on six experiments, and error bars indicate the standard error of the mean.
Protein identification.
Table 1 shows the proteins identified by these methods. All 18 of these proteins are found in both the 26695 and J99 strains of H. pylori. Many of these proteins were previously classified only as hypothetical H. pylori proteins, and these are identified here by gene sequence. One protein was VacA (HP0887), and the others were a conserved secreted protein (HP1286), a putative peptidyl cis-trans isomerase (HP0175), six proteins encoded by HP0305, HP0231, HP0973, HP0721, HP0129, and HP0902, thioredoxin (HP1458), single-stranded-DNA-binding 12RNP2 precursor (HP0827), histone-like DNA-binding protein HU (HP0835), ribosomal protein L11 (HP1202), a putative outer membrane protein (HP1564), and two outer membrane proteins, Omp21 (HP0913) and Omp20 (HP0912). The two other proteins with a ratio of around 7 were ribosomal protein L1 (HP1201) and a protein of unknown function encoded by HP0720.
TABLE 1.
Identities of enriched proteins in the supernatant determined by using mass spectrometry
| Spot no. | GenBank accession no. | Encoding gene (protein name) | Size (kDa) | pIa | Signal peptideb |
|---|---|---|---|---|---|
| 1 | NP_207680 | HP0887 (VacA) | 87c | 9.37 | Yes |
| 2 | NP_208078 | HP1286 (conserved secreted protein) | 20.6d | 9.37 | Yes |
| 3 | P56112 | HP0175 (putative peptidyl cis-trans isomerase in J99) | 34.0d | 9.58 | Yes |
| 4 | NP_207103 | HP0305 (putative human regulator of G protein signaling 12) | 20.0d | 9.73 | Yes |
| 5 | NP_207029 | HP0231 | 29.5d | 9.35 | Yes |
| 6 | NP_207764 | HP0973 | 39.8d | 9.69 | Yes |
| 7 | NP_207515 | HP0721 | 17.6d | 8.42 | Yes |
| 8 | NP_206929 | HP0129 | 16.2d | 9.51 | Yes |
| 9 | NP_207695 | HP0902 | 11.0 | 7.50 | No |
| 10 | NP_208249 | HP1458 (thioredoxin) | 11.7 | 8.17 | No |
| 11 | NP_207620 | HP0827 (ssDNA-binding 12RNP2 precursor) | 9.4 | 9.71 | No |
| 12 | NP_207628 | HP0835 (histone-like DNA-binding protein HU) | 10.4 | 9.74 | No |
| 13 | NP_207993 | HP 1202 (ribosomal protein L11) | 15.3 | 10.28 | No |
| 14 | NP_208355 | HP1564 (putative outer membrane protein in J99) | 30.2d | 9.29 | Yes |
| 15 | NP_207705 | HP0913 | 57.1d | 8.96 | Yes |
| 16 | NP_207704 | HP0912 | 55.9d | 9.18 | Yes |
| 17 | NP_207992 | HP1201 (ribosomal protein L1) | 25.3 | 10.40 | No |
| 18 | NP_207514 | HP0720 | 6.1 | 8.93 | No |
Isoelectric point of each protein, calculated after removal of the signal peptide.
Defined by Signal V1.1 World Wide Web Server and iPSORT.
Cytotoxin size after removal of the signal peptide (33 amino acids) and C-terminal autotransporter (50 kDa).
Expected size, including variably sized (14 to 41 amino acids) signal peptide.
Sixteen of these 18 proteins (Fig. 2) had spots matching both their expected isoelectric point and molecular size. The predicted molecular size of the protein encoded by HP0973, including its signal peptide (28 amino acids), was 39.8 kDa, but that of this protein in the two-dimensional gel was around 23 to 25 kDa, indicating either that this protein was partially degraded prior to electrophoresis or that the protein may have an unusual mobility during SDS-PAGE. The molecular size of the protein encoded by HP0720 was 6.1 kDa, but that of this protein in the two-dimensional gel was around 14 kDa. This might be due to the formation of a dimer or an electrophoretic abnormality as a result of a high proportion of basic amino acids in this low-molecular-weight protein, as has been observed previously for histones (38).
All of these proteins except five, protein HP0902, thioredoxin, single-stranded-DNA-binding 12RNP2 precursor, histone-like DNA-binding protein, and ribosomal protein L11, were found to have a signal peptide by at least two algorithms (Signal V1.1 World Wide Web Server [33] and iPSORT [4]) based on the degree of hydrophobicity in the N terminus. Among these proteins with a signal peptide, several had regions of homology with known functional proteins. Protein HP0175 appeared to be a peptidyl prolyl cis-trans isomerase with strong homology to this family of enzymes in other bacteria, such as Bacillus subtilis. Protein HP0305 contained a domain in its middle third with 45% homology to a regulator of human G protein signaling protein 12. Protein HP0231 also contained a domain 51% homologous to a thiol-disulfide interchange protein in Ralstonia solanacea. The other proteins of this type, HP1286, HP0973, HP0721, and HP0129, were not classified by a Blast search.
Five proteins enhanced in the supernatant (protein HP0902, thioredoxin, single-stranded-DNA-binding 12RNP2 precursor, histone-like DNA-binding protein, and ribosomal protein L11) did not have a signal peptide. Protein HP0902 contained a domain 56% homologous to acetate kinase in Streptococcus agalactiae. Thioredoxin contained a reversibly reducible cystine disulfide group that could participate in an antioxidant system. Single-stranded-DNA-binding 12RNP2 precursor had 72% homology to single-stranded-DNA-binding 12RNP2 precursor in a Synechococcus sp. It also showed homology (70%) with a human polyadenylation protein, CSTF-64, indicating the existence of a eukaryotic ribonucleoprotein consensus sequence-type RNA-binding protein in a prokaryote (45).
Protein HP1564, which has been classified as a putative outer membrane protein in J99, is predicted to have a signal peptide and had homology (76%) to an ABC transporter substrate-binding protein of a Fusobacterium nucleatum subspecies and homology (66%) to a probable TonB-dependent receptor (PA5505) in Pseudomonas aeruginosa. Two H. pylori outer membrane proteins, Omp20 (HP0912) and Omp21 (HP0913), had strong homology with known outer membrane proteins, and these proteins have previously been designated alpA/hopA and alpB/hopB, respectively.
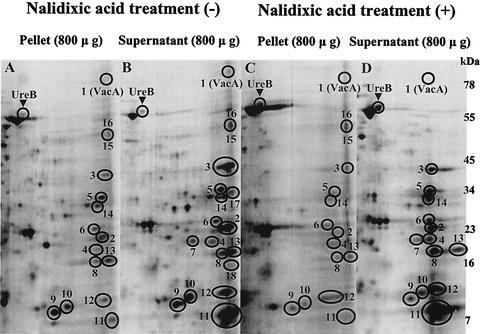
The presence of some outer membrane proteins suggested that outer membrane shedding during cell division could be responsible for their release into the supernatant. When cultures were treated with 100 μg of nalidixic acid per ml during labeling with the assumption that nalidixic acid, an inhibitor of cell division but not protein synthesis, might have some role in the prevention of membrane shedding, the outer membrane proteins Omp21 and Omp20, which had been enhanced in the supernatant prior to nalidixic acid treatment (Fig. 4B), were no longer present in the supernatant (Fig. 4D). VacA also decreased with nalidixic acid treatment (Fig. 4D), consistent with the previous finding that VacA release is dependent not only on autotransporter-related secretion but also on outer membrane shedding during cell division (23). In contrast, 13 of the 16 proteins were still more than 10-fold enriched in the supernatant (Fig. 4D) compared to the pellet (Fig. 4C). The ribosomal protein L1 and protein HP0720, with an enrichment ratio near 7.0 (Fig. 3), and thus with low probability of being secreted proteins, were also absent after nalidixic acid treatment (Fig. 4D).
FIG. 4.
Comparison between phosphorimages of two-dimensional gel electrophoresis of the pellet (A) and supernatant (B) without nalidixic acid (100 μg/ml) treatment and of the pellet (C) and supernatant (D) after nalidixic acid treatment. Thirteen proteins were still enriched in the supernatant (D) compared with the pellet (C) after nalidixic acid treatment. In contrast, VacA (1) was much decreased, and two outer membrane proteins, Omp21 (15) and Omp20 (16), vanished in the supernatant after nalidixic acid treatment.
DISCUSSION
H. pylori is able both to secrete proteins and also to release cytoplasmic proteins due to lysis. Two well-known pathogenic proteins that are secreted are VacA and CagA. However, the high frequency of asymptomatic VacA+ CagA+ H. pylori carriers and the lack of association between VacA and CagA status and clinical outcome (39, 52) suggest that there may be other secreted proteins also related to inflammation.
There have been several attempts to identify proteins secreted by H. pylori (8, 43, 49). Schraw et al. (43) found that many proteins that were components of intact bacterial cells and therefore resulted from nonselective release of proteins by lysis accumulated in culture supernatants. On the other hand, VacA (87 kDa) was detected only in trace quantities in the bacterial cell pellet but appeared as a major extracellular protein in the supernatant after 24 h. Additional proteins also appeared in the supernatant due to lysis. Marcus and Scott (30) found that extracellular urease appears in the supernatant of H. pylori cultures proportionally with green fluorescent protein (GFP) expressed in these cells, establishing the lytic nature of urease release. Hence, high levels of H. pylori lysis have confounded previous attempts at defining specifically released proteins.
To avoid contamination due to H. pylori lysis, the supernatant was separated from intact bacteria by a low- and a high-speed centrifugation step on a 10% Ficoll step gradient to cleanly separate cells and cell fragments from released soluble proteins. With this two-step centrifugation method, HopB, a qualitative indicator of outer membrane protein breakage (Fig. 1B), and UreI, an indicator of inner membrane protein breakage (Fig. 1C), were visible in the pellet but not in the supernatant, indicating that contamination by either outer or inner membrane proteins had been reduced with this procedure. Pulse-labeling with [35S]methionine and two-dimensional gel electrophoresis made the search for secreted proteins easier by identifying only proteins that were synthesized and then released during the 4-h labeling period and ignoring proteins released previously by autolysis.
In spite of these precautions, the mean ratio of UreB in the supernatant over that in the pellet was 0.25, indicating that proteins released by lysis during the 4-h pulse-chase still contaminated the supernatant. Values much greater than the UreB ratio can identify those proteins that are released into the medium due to a process other than lysis, i.e., secretion. Proteins were considered candidates for specific release only if their enrichment factor, obtained by comparing levels found in the supernatant and in the pellet in comparison to UreB, was greater than 10. Sixteen proteins initially satisfied this criterion (Fig. 3).
Of these 16 proteins, two outer membrane proteins, Omp21 and Omp20, were enriched in the supernatant. These may be shed during cell division and contaminate the isolated supernatant of H. pylori, probably another confounding factor in identifying secreted proteins. When the bacteria were treated with 100 μg of nalidixic acid, which inhibits DNA synthesis and cell division without impairing RNA and protein synthesis (20), per ml, Omp21 and Omp20 vanished from the supernatant (Fig. 4D). In addition, VacA was also reduced. The latter must incorporate into the outer membrane during the autotransport process (42). In contrast, the other 13 proteins still remained enhanced in the supernatant after nalidixic acid treatment (Fig. 4D). Hence, the selective action of inhibition of DNA synthesis by nalidixic acid on the apparent secretion of these three proteins known to enter the outer membrane indicates that these are released as a function of outer membrane elaboration (23). There may be other, as yet undetermined mechanisms involved in nalidixic acid action, but it seems that the prevention of membrane shedding during replication is a reasonable explanation for its selective action.
There are at least five pathways for secretion of bacterial proteins (12, 25). Sec-dependent (type II and autotransport) secretion requires an amino-terminal hydrophobic signal (or leader) peptide that is cleaved after translocation across the inner membrane (37). The secreted protein is folded in the periplasmic space and may undergo further modification, such as disulfide bond formation or subunit assembly, before translocation across the outer membrane via the secretion apparatus in the case of type II secretion (41) or via a pore (β-barrel) which is made up of the C-terminal segment of secreted protein precursor in the case of autotransport secretion, such as VacA (42).
Type I and type III secretory systems are independent of the Sec system because they do not contain a signal peptide and utilize alternative pathways that facilitate coherent translocation of proteins across both the inner and outer membranes into the medium or into adjacent eukaryotic cells. The type III injection machinery requires approximately 20 proteins, whereas the type I machinery requires at least three (25), but there does not appear to be a type I or type III secretory system in the H. pylori genome (47). Instead, H. pylori has been shown to possess type IV secretion machinery, which can translocate proteins both across bacterial membranes and then across the plasma membrane of the eukaryotic cell to which it is attached, independent of the Sec system (12). CagA, a representative of this type IV secreted protein (12), was not enhanced in the supernatant, supporting the concept that contact with eukaryotic cells is necessary for its secretion (2, 12, 35).
Of the 13 proteins showing a greater than 10-fold enrichment in the absence of cell division, 8 were predicted to have signal peptides (Table 1). These proteins, HP1286, HP0175, HP0305, HP0231, HP0973, HP0721, HP0129, and HP1564, were too small to contain the multiple domains necessary for autotransport (42). If these eight proteins are indeed secreted, they are likely to reflect type II secretion, moving out via the general secretory pathway, the primary pathway for the secretion of extracellular degradative enzymes by gram-negative bacteria (25).
Protein HP0175 is likely to be peptidyl-prolyl cis-trans isomerase (a protein chaperone [44]) and is one of five antigens of H. pylori preferentially recognized by the antibodies of patients with gastroduodenal ulcers rather than nonulcer dyspepsia patients (1). Protein HP0305, of unknown function in H. pylori, contains a domain of homology to the human regulator of G protein signaling 12. Regulators of G protein signaling are a relatively newly described family of eukaryotic proteins that attenuate G protein-mediated pathways by acting as GTPase-activating proteins for Gα subunits (40), suggesting that protein HP0305 might have an effect on the G protein-transmitted signal pathway of host cells if it gets into host cells. Another protein, HP0231, of unknown function in H. pylori has homology to thiol-disulfide interchange protein, suggesting the possibility of involvement in disulfide interchange reactions, like thioredoxin.
Protein HP0902, thioredoxin, single-stranded-DNA-binding 12RNP2 precursor, histone-like DNA-binding protein HU, and ribosomal protein L11 do not have a signal peptide (Table 1), and thus it is not clear by what mechanism they might be secreted. However, the finding that their enrichment ratio relative to UreB was also more than 10-fold, even after nalidixic acid treatment, suggests that these five proteins are specifically released or secreted.
Thioredoxin reduces oxidized proteins via disulfide exchange. Such antioxidant systems are critical to the defense of H. pylori against reactive oxygen species generated by the oxidative burst of macrophages and polymorphonuclear leukocytes (3), suggesting that thioredoxin (HP1458) may play a protective role for H. pylori in the gastric tissue. Single-stranded-DNA-binding 12RNP2 precursor shows homology with human polyadenylation protein CSTF-64. It is known that polyadenylation factors link nuclear polyadenylation to a variety of cellular processes and that they can be important targets for regulating gene expression (10). Similarly, histone-like DNA-binding protein HU, a nonspecific histone-like DNA-binding protein, is known to participate in a number of genomic events as an accessory protein and has been shown to bind specifically to DNA (51).
Recently, Bumann et al. (7) published a proteomic analysis of the secreted proteins of H. pylori, and five (VacA, HP1286, HP0175, HP0231, and HP1458) of our 14 proteins, including VacA, were also found among the 19 proteins that they identified. The variation in data might result from methodological differences. These investigators used brain heart infusion broth and incubated the cells for 20 h, in contrast to the 4-h incubation and the RPMI-based methionine-free minimal medium 1640 containing [35S]methionine used here. Also, they collected the supernatant by filtration through 0.45-μm-pore-size membranes to remove residual bacteria and precipitated it with trichloroacetic acid. This method would not remove proteins released by lysis over the 20-h incubation time, in contrast to the step gradient used here. However, these two studies are complementary in defining the secreted proteins of H. pylori.
A novel method of identifying proteins of H. pylori released or secreted into the medium as opposed to those released by general lysis is described here. This method identified VacA, the only H. pylori protein previously known to be released into the medium, as being secreted, whereas the cytoplasmic proteins UreB and Hsp60 were shown to be released only by nonspecific cell lysis. This technique should be applicable to the study of protein secretion in other highly autolytic bacteria. In addition to VacA, 13 proteins were found to be selectively enriched in the medium and thus were specifically released, possibly by secretion or selective loss from the periplasm. Some of these proteins may have as yet unknown roles in H. pylori-induced inflammation or pathogenesis.
Acknowledgments
This work was supported by USVA and NIH grants DK46917, 53462, 41301, and 17294.
REFERENCES
- 1.Atanassov, C., L. Pezennec, J. d'Alayer, G. Grollier, B. Picard, and J.-L. Fauchere. 2002. Novel antigens of Helicobacter pylori correspond to ulcer-related antibody pattern of sera from infected patients. J. Clin. Microbiol. 40:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backert, S., E. Ziska, V. Brinkmann, U. Zimny-Arndt, A. Fauconnier, P. R. Jungblut, M. Naumann, and T. F. Meyer. 2000. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2:155-164. [DOI] [PubMed] [Google Scholar]
- 3.Baker, L. M. S., A. Raudonikiene, P. S. Hoffman, and L. B. Poole. 2001. Essential thioredoxin-dependent peroxiredoxin system from Helicobacter pylori: genetic and kinetic characterization. J. Bacteriol. 183:1961-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannai, H., Y. Tamada, O. Maruyama, K. Nakai, and S. Miyano. 2002. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 18:298-305. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J. 1992. Hypothesis on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology 102:720-727. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J., and J. Parsonnet. 1994. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J. Clin. Investig. 94:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen. Infect. Immun. 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, P., M. S. McClain, M. H. Forsyth, and T. L. Cover. 1998. Extracellular release of antigenic proteins by Helicobacter pylori. Infect. Immun. 66:2984-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, X. G., P. Correa, J. Offerhaus, E. Rodriguez, F. Janney, E. Hoffmann, J. Fox, and S. Diavolitsis. 1986. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am. J. Clin. Pathol. 86:575-582. [DOI] [PubMed] [Google Scholar]
- 10.Coglan, D. F., and J. L. Manley. 1997. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 11:2755-2766. [DOI] [PubMed] [Google Scholar]
- 11.Covacci, A., S. Censini, M. Bugnoli, R. Petracca, D. Burroni, G. Macchia, A. Massone, E. Papini, Z. Xiang, N. Figura, and R. Rappuoli. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 90:5791-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 13.Cover, T. L., C. P. Dooley, and M. J. Blaser. 1990. Characterization and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolating cytotoxin activity. Infect. Immun. 58:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cover, T. L., P. Cao, C. D. Lind, K. T. Tham, and M. J. Blaser. 1993. Correlation between vacuolating cytotoxin production by Helicobacter pylori isolates in vitro and in vivo. Infect. Immun. 61:5008-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cover, T. L., and M. J. Blaser. 1996. Helicobacter pylori infection, a paradigm for chronic mucosal inflammation: pathogenesis and implications for eradication and prevention. Adv. Intern. Med. 41:85-117. [PubMed] [Google Scholar]
- 16.Crabtree, J. E., J. D. Taylor, J. I. Wyatt, R. V. Heatley, T. M. Shallcross, D. S. Tompkins, and B. J. Rathbone. 1991. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet 338:332-335. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree, J. E., T. M. Shallcross, R. V. Heatley, and J. I. Wyatt. 1991. Mucosal tumor necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 32:1473-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabtree, J. E., P. Peichl, J. I. Wyatt, U. Stachl, and I. J. D. Lindley. 1993. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand. J. Immunol. 37:65-70. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree, J. E., J. I. Wyatt, L. K. Trejdosiewicz, P. Peichl, P. H. Nichols, N. Ramsay, J. N. Primrose, and I. J. D. Lindley. 1994. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J. Clin. Pathol. 47:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis, B. D., R. Dulbecco, H. N. Eisen, H. S. Ginsberg, and W. B. Wood, Jr. 1968. Principles of microbiology and immunology, p. 327. Harper & Row Publishers, New York, N.Y.
- 21.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Exner, M. M., P. Doig, T. J. Trust, and R. E. W. Hancock. 1995. Isolation and characterization of a family of porin proteins from Helicobacter pylori. Infect. Immun. 63:1567-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiocca, R., V. Necchi, P. Sommi, V. Ricci, J. Telford, T. L. Cover, and E. Solicia. 1999. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188:220-226. [DOI] [PubMed] [Google Scholar]
- 24.Hessey, S. J., J. Spencer, J. I. Wyatt, G. Sobala, B. J. Rathbone, A. T. Axon, and M. F. Dixon. 1990. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut 31:134-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazi, J. L., R. Sinniah, V. Zaman, M. L. Ng, N. A. Jafarey, S. M. Alam, S. J. Zuberi, and A. M. Kazi. 1990. Ultrastructural study of Helicobacter pylori-associated gastritis. J. Pathol. 161:65-70. [DOI] [PubMed] [Google Scholar]
- 27.Labigne, A., and H. de Reuse. 1996. Determinants of Helicobacter pylori pathogenicity. Infect. Agents Dis. 5:191-202. [PubMed] [Google Scholar]
- 28.Lee, A., J. Fox, and S. Hazell. 1993. Pathogenicity of Helicobacter pylori: a perspective. Infect. Immun. 61:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leunk, R. D., P. T. Johnson, B. C. David, W. G. Kraft, and D. R. Morgan. 1988. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J. Med. Microbiol. 26:93-99. [DOI] [PubMed] [Google Scholar]
- 29a.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 30.Marcus, E. A., and D. R. Scott. 2001. Cell lysis is responsible for the appearance of extracellular urease in Helicobacter pylori. Helicobacter 6:93-99. [DOI] [PubMed] [Google Scholar]
- 31.Mobley, H. L. 1997. Helicobacter pylori factors associated with disease development. Gastroenterology 113(Suppl. 6):S21-S28. [DOI] [PubMed] [Google Scholar]
- 32.Munzenmaier, A., C. Lange, E. Glocker, A. Covacci, A. Moran, S. Bereswill, P. A. Baeuerle, M. Kist, and H. L. Pahl. 1997. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-κB. J. Immunol. 159:6140-6147. [PubMed] [Google Scholar]
- 33.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Noach, L. A., N. B. Bosma, J. Jansen, F. J. Hoek, S. J. H. van Deventer, and G. N. J. Tytgat. 1994. Mucosal tumor necrosis factor-α, interleukin-1β, and interleukin-8 production in patients with Helicobacter pylori infection. Scand. J. Gastroenterol. 29:425-429. [DOI] [PubMed] [Google Scholar]
- 35.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 36.Oppenheim, J. J., C. O. C. Zachariae, N. Mukaiada, and K. Matsushima. 1991. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu. Rev. Immunol. 9:617-648. [DOI] [PubMed] [Google Scholar]
- 37.Paetzel, M., R. E. Dalbey, and N. C. Strynadka. 2000. The structure and mechanism of bacterial type I signal peptidases. A novel antibiotic target. Pharmacol. Ther. 87:27-49. [DOI] [PubMed] [Google Scholar]
- 38.Panyim, S., and R. Chalkley. 1971. The molecular weights of vertebrate histones exploiting a modified sodium dodecyl sulfate electrophoretic method. J. Biol. Chem. 25:7557-7560. [PubMed] [Google Scholar]
- 39.Park, S. M., J. Park, J. G. Kim, H. D. Cho, J. H. Cho, D. H. Lee, and Y. J. Cha. 1998. Infection with Helicobacter pylori expressing the cagA gene is not associated with an increased risk of developing peptic ulcer diseases in Korean patients. Scand. J. Gastroenterol. 33:923-927. [DOI] [PubMed] [Google Scholar]
- 40.Ross, E. M., and T. M. Wilkie. 2000. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69:795-827. [DOI] [PubMed] [Google Scholar]
- 41.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt, W., and R. Haas. 1994. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol. Microbiol. 12:307-319. [DOI] [PubMed] [Google Scholar]
- 43.Schraw, W., M. S. McClain, and T. L. Cover. 1999. Kinetics and mechanisms of extracellular protein release by Helicobacter pylori. Infect. Immun. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiene-Fischer, C., and C. Yu. 2001. Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. 495:1-6. [DOI] [PubMed] [Google Scholar]
- 45.Sugita, M., and M. Sugiura. 1994. The existence of eukaryotic ribonucleoprotein consensus sequence-type RNA-binding proteins in a prokaryote, Synechococcus 6301. Nucleic Acids Res. 22:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taub, D. D., and J. J. Oppenheim. 1993. Review of the chemokine meeting “The Third International Symposium of Chemotactic Cytokines.” Cytokine 5:175-179. [DOI] [PubMed] [Google Scholar]
- 47.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelly, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 48.Tummuru, M. K. R., T. L. Cover, and M. J. Blaser. 1993. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect. Immun. 61:1799-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanet, A., and A. Labigne. 1998. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect. Immun. 66:1023-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weeks, D. L., S. Eskandari, D. R. Scott, and G. Sachs. 2000. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 287:482-485. [DOI] [PubMed] [Google Scholar]
- 51.Wojtuszewski, K., M. E. Hawkins, J. L. Cole, and I. Mukerji. 2001. HU binding to DNA: evidence for multiple complex formation and DNA bending. Biochemistry 40:2588-2598. [DOI] [PubMed] [Google Scholar]
- 52.Yamaoka, Y., T. Kodama, O. Gutierrez, J. G. Kim, K. Kashima, and D. Y. Graham. 1999. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J. Clin. Microbiol. 37:2274-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]