Abstract
Iron scavenging by Neisseria gonorrhoeae is accomplished by the expression of receptors that are specific for host iron-binding proteins, such as transferrin and lactoferrin. Efficient transferrin-iron acquisition is dependent on the combined action of two proteins, designated TbpA and TbpB. TbpA is a TonB-dependent outer membrane receptor, whereas TbpB is lipid modified and serves to increase the efficiency of transferrin-iron uptake. Both proteins, together or separately, can be isolated from the gonococcal outer membrane by using affinity chromatography techniques. In the present study, we identified an additional protein in transferrin-affinity preparations, which had an apparent molecular mass of 45 kDa. The ability to copurify this protein by transferrin affinity was dependent upon the presence of TbpA and not TbpB. The amino-terminal sequence of the 45-kDa protein was identical to the amino terminus of gonococcal TonB, indicating that TbpA stably interacted with TonB, without the addition of chemical cross-linkers. Using immunoprecipitation, we could recover TbpA-TonB complexes without the addition of transferrin, suggesting that ligand binding was not a necessary prerequisite for TonB interaction. In contrast, a characterized TonB box mutant of TbpA did not facilitate interaction between these two proteins such that complexes could be isolated. We generated an in-frame deletion of gonococcal TonB, which removed 35 amino acids, including a Neisseria-specific, glycine-rich domain. This mutant protein, like the parental TonB, energized TbpA to enable growth on transferrin. Consistent with the functionality of this deletion derivative, TbpA-TonB complexes could be recovered from this strain. The results of the present study thus begin to define the requirements for a functional interaction between gonococcal TbpA and TonB.
Bacterial pathogens infecting humans must propagate in an environment that maintains a very small reservoir of soluble iron (38), and since iron is required for the growth of nearly all microorganisms, efficient iron scavenging systems are essential. Most bacteria acquire this scarce but necessary nutrient by synthesis of low-molecular-weight siderophores in addition to membrane-bound transporters for ferric-siderophore uptake (35). However, the pathogenic Neisseria species are not known to secrete iron-scavenging siderophores (46) but have evolved outer membrane receptors that directly bind to host iron sources, such as transferrin and lactoferrin, and relieve them of their bound iron. The transferrin receptor is composed of two proteins: an integral outer membrane, TonB-dependent receptor (TbpA), and a surface-exposed lipoprotein (TbpB) (4, 15, 17). Together, these proteins specifically bind human transferrin and remove and internalize the iron in an energy-dependent fashion (13, 16). By analogy with the well-defined activities of the TonB-dependent siderophore receptors, we proposed that receptor-dependent iron removal from transferrin is accomplished at the expense of the cytoplasmic membrane proton motive force, transduced through TonB to TbpA (13, 17).
This process of iron acquisition from transferrin by a TonB-dependent receptor is analogous to the process of siderophore internalization in Escherichia coli (for a recent review, see reference 36) in that TonB is required for ligand transport across the outer membrane. TonB homologues have been identified in more than 25 different gram-negative bacteria, a finding consistent with an effective and well-conserved mechanism for energization of outer membrane receptors. TonB is largely a periplasmic protein with an uncleaved leader peptide, with which it is anchored to the cytoplasmic membrane (37). This hydrophobic amino-terminal tether is important for the function of TonB since it not only acts as an anchor but is also required for the interaction between TonB and the ExbB/D complex localized in the cytoplasmic membrane (22, 23). The multimeric ExbB/D complex has been shown to stabilize TonB (32) and is proposed to be a proton pump that harnesses the proton motive force generated across the cytoplasmic membrane (21).
Evidence of a physical interaction between TonB and outer membrane receptors was inferred from the finding that overexpressed FhuA could inhibit the degradation of overexpressed TonB (19). Subsequent experiments using in vivo formaldehyde cross-linking demonstrated an association between TonB and the siderophore receptor, FepA (27-29, 43). These findings were supported by results of site-directed disulfide cross-linking experiments, which confirmed a physical, highly specific interaction between BtuB and TonB (9, 10). Recently, an in vitro interaction between His-tagged TonB and the purified outer membrane receptors FepA and FhuA has been reported (34). A current model of TonB function suggests that the protein is charged at the cytoplasmic membrane; it shuttles across the periplasm and subsequently interacts, via its carboxy terminus, with the outer membrane receptors where energy is expended for ligand transport (29, 32).
A region of homology near the amino termini of TonB-dependent receptors has been termed the TonB box. Specific mutations in this five-residue domain that convert hydrophobic residues to turn-promoting amino acids have been shown in several systems to render the receptor deenergized and insensitive to TonB (5, 18, 19, 39). Genetic evidence for a direct, physical interaction between TonB-dependent receptors and TonB was implied by the isolation of extragenic mutations in E. coli TonB that compensated for the TonB-box defect (5, 13, 18-20, 39). Although these compensatory mutations were sufficient to rescue the TonB-box mutation, recent evidence indicates that the suppression was not complete (12). Coggshall et al. suggest that the compensatory mutation in TonB allowed for a single cycle of receptor charging but that the original TonB-box mutation prevented subsequent rounds of uptake (12). We created a similar TonB-box mutation in gonococcal TbpA, which likewise generated a binding-competent, uptake-defective transferrin receptor (13). This mutant bound ligand with extremely high affinity, a result which was likely due, at least in part, to the observed impaired release of transferrin (13).
In the present study, we identified a 45-kDa protein that specifically co-affinity purified with gonococcal TbpA. This protein could be coimmunoprecipitated with TbpA by using a TbpA-specific antibody, even in the absence of added ligand (transferrin). We found that this 45-kDa protein was highly immunogenic and that its expression was iron regulated. Amino-terminal sequence analysis of the copurifying protein revealed that it was gonococcal TonB, a finding consistent with a stable interaction between this protein and the outer membrane protein it energizes. We generated TonB mutants and analyzed their ability to interact with TbpA in addition to determining the effect of a characterized TonB-box mutation in TbpA on TbpA-TonB interaction.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The gonococcal strains used in the present study are listed in Table 1. Bacteria were maintained on GCB medium (Difco) containing Kellogg's supplement (24) and 12 μM Fe(NO3)3. Plates were incubated at 37°C in a 5% CO2 atmosphere. For large-scale growth of gonococcal strains, nonpiliated gonococci were grown in GC broth containing Kellogg's supplement at 35°C in a 5% CO2 atmosphere with vigorous shaking. Desferal (desferoxamine mesylate; Sigma Aldrich) was added to a final concentration of 150 μM to induce iron stress. For iron-replete controls, Fe(NO3)3 was added to a final concentration of 12 μM. Iron-stressed and iron-replete cells were harvested after 5 h of growth.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotypea | Relevant phenotype | Source or reference |
|---|---|---|---|
| Strain | |||
| FA19 | Wild type | 33 | |
| FA6839 | Lbp− | 6 | |
| MCV601 | FA19 lbpB::Ω (Strr Spcr) | TbpA+ TbpB+ Lbp− | This study |
| FA6747 | FA19 tbpA::mTn3 (Cmr) | TbpA− TbpB+ | 15 |
| MCV602 | FA19 tbpA::mTn3 (Cm) lbpB::Ω (Strr Spcr) | TbpA− TbpB+ Lbp− | This study |
| FA6905 | FA19 ΔtbpB | TbpA+ TbpB− | 16 |
| MCV603 | FA19 ΔtbpB lbpB::Ω (Strr Spcr) | TbpA+ TbpB− Lbp− | This study |
| FA6819 | FA19 ΔtbpB | TbpA+ TbpB− | 4 |
| FA6815 | FA19 tbpB::Ω (Strr Spcr) | TbpA− TbpB− | 4 |
| FA6935 | FA19 tbpA | TbpA (I16P) TonB box negative | 13 |
| MCV604 | FA19 tbpA lbpB::Ω (Strr Spcr) | TbpA (I16P) TonB box negative, Lbp− | This study |
| MCV607 | FA19 tonB lbpB::Ω (Strr Spcr) | TonBΔ160-194, Lbp− | This study |
| MCV609 | FA19 tonBΩ (Genr) lbpB::Ω (Strr Spcr) | TonB− Lbp− | This study |
| Plasmid | |||
| pCR2.1 | Ampr Kanr | Invitrogen | |
| pVCU689 | pCR2.1 containing wild-type tonB gene from strain MCV601 | This study | |
| pVCU690 | pCR2.1 containing tonBΔ160-194 allele | This study | |
| pVCU691 | pCR2.1 containing tonBΩ allele | This study |
Strr, streptomycin resistance; Spcr, spectinomycin resistance; Ampr, ampicillin resistance; Kanr, kanamycin resistance.
The plasmids described in Table 1 were propagated in E. coli TOP10 (Invitrogen). For cloning and mutagenesis, Luria-Bertani (Difco) and GCB plates were supplemented with ampicillin (100 μg/ml; Sigma), streptomycin (100 μg/ml; Sigma), or gentamicin (15 μg/ml for E. coli, 5 μg/ml for N. gonorrhoeae; Life Technologies) as needed for selection of transformants.
Recombinant DNA techniques.
Plasmid isolation, restriction digests, and ligations were carried out according to the manufacturers' recommendations. Restriction endonucleases, Taq DNA polymerase, and concentrated T4 DNA ligase were purchased from Life Technologies. Oligonucleotide primers were synthesized by Integrated DNA Technologies (Coralville, Iowa). Full-length gonococcal tonB was PCR amplified from the chromosome of strain MCV601 (Table 1) by using the forward primer oVCU142 (5′-GCCTATGAATGGAGCAGGC-3′) and the reverse primer oVCU143 (5′-CAGGACGGGATCGCCCG-3′). The 1,046-bp PCR product generated by this reaction was isolated and ligated into pCR2.1 TOPO (Invitrogen) generating pVCU689 (Table 1). To mutagenize tonB, pVCU689 was digested with AgeI, the recognition site for which occurs twice within tonB but is not present elsewhere in the plasmid. The linearized plasmid, lacking the 105-bp AgeI fragment, was excised from the gel and religated to yield pVCU690 (Table 1). Insertion of the Ω gentamicin cassette into mutagenized tonB was carried out by digesting pVCU690 with PinAI (an isoschizomer of AgeI), followed by treatment of the plasmid with Klenow fragment to generate blunt ends. A 1.6-kb MluI fragment of pBSL142 (3) containing the Ω cassette was similarly treated prior to ligation with the linearized pVCU690. The resulting plasmid was designated pVCU691 (Table 1).
Gonococcal transformation.
Lbp− derivatives of gonococcal strains FA19, FA6747, FA6905, and FA6935 were generated in order to eliminate cross-reactivity between anti-TbpA sera and a similar, TonB-dependent receptor, LbpA (15). Chromosomal DNA from strain FA6839 (kindly provided by Gour Biswas) was transformed into gonococcal strain FA19 as described previously (4). The tonB deletion mutation was moved into the gonococcal chromosome by simultaneous transformation of chromosomal DNA from FA6839 and linearized plasmid pVCU690 into piliated FA19, selecting for streptomycin resistance and screening for the presence of the tonB deletion by PCR. The tonBΩ knockout mutant was constructed by transformation of piliated gonococcal strain FA19 with DNA from plasmid pVCU691, selecting for gentamicin resistance, followed by transformation with chromosomal DNA from strain FA6839, selecting for streptomycin resistance.
Affinity purification of gonococcal transferrin-binding proteins.
For analytical, small-scale analysis, batch purifications were performed essentially as described previously (16). For purification of TbpA from FA19 (TbpA+ TbpB+; Table 1) or FA6819 (TbpA+ TbpB−; Table 1), proteins were solubilized in 0.5% Sarkosyl, and the matrix was washed in 1 M NaCl. To purify TbpB in the absence of TbpA, from strain FA6747 (TbpA− TbpB+; Table 1), lower-ionic-strength conditions were required as follows: 0.1% Triton X-100 was used for solubilization, and the matrix was washed in 500 mM NaCl. Binding proteins were eluted with 1% β-mercaptoethanol in the above washing buffer. Specificities of binding reactions were assessed by performing mock purifications in the absence of biotinylated transferrin.
For large-scale purifications of TbpA and TbpA+TbpB to be used as immunogens, a modification of the above procedure was developed. A total of 30 mg of total membrane proteins from iron-stressed cultures were solubilized in 2% Elugent (Calbiochem), 10 mM EDTA, and 0.5% Sarkosyl; insoluble material was removed by centrifugation at 27,000 × g. Ferrated, human transferrin (Calbiochem) was conjugated to Affigel 15 (Bio-Rad) and used as the affinity matrix. The transferrin-conjugated affinity matrix was mixed with solubilized membrane proteins and incubated overnight. The matrix was loaded into a disposable column and then washed with 20 bed volumes of phosphate-buffered saline containing 2% Elugent and 1 M NaCl. The bound proteins were eluted by the addition of 50 mM glycine (pH 2.5) containing 1.0% octylglucoside (n-octyl-β-d-glucopyranoside; Calbiochem) and immediately neutralized by elution into 1.0 M Tris (pH 8.0). Protein purified in this manner retained transferrin-binding capabilities, as assessed by solid-phase HRP-Tf binding assay (16) (data not shown). The energy transduction system mutants were evaluated in an affinity purification procedure similar to the large-scale purification described above except that membrane proteins were solubilized in phosphate-buffered saline containing 1% Elugent and 10 mM EDTA with no Sarkosyl. The matrix was washed with phosphate-buffered saline containing 1% octylglucoside and eluted as described above.
Immunoprecipitation.
Total membrane proteins from gonococcal strains MCV601, MCV602, and MCV603 (Table 1) were solubilized in phosphate-buffered saline containing 1% Elugent (Calbiochem) and 10 mM EDTA. Proteins were ultracentrifuged at 100,000 × g, and the resulting supernatant was incubated with polyclonal rabbit serum raised against recombinant TbpA (rTbpA) (45) from Neisseria meningitidis strain SD (2) (kindly provided by Andrew Gorringe). Protein A-agarose beads (Calbiochem) were added and allowed to bind, after which the agarose beads were pelleted and the supernatant was removed for analysis. The pellet was washed in phosphate-buffered saline, and the resulting bound proteins were resuspended in Laemmli solubilizing buffer containing 2% β-mercaptoethanol (26) for analysis in parallel with the supernatant or unbound fractions.
Polyclonal antisera.
In an attempt to generate polyclonal antisera specific for TbpA alone and for TbpA+TbpB, we immunized mice with affinity-purified proteins isolated from gonococcal strains FA19 and FA6905 (Table 1) as described above. For the TbpA immunizations, 10 mice were vaccinated subcutaneously in the scruff of the neck. Animals received an initial immunization of 10 μg of protein, purified via the large-scale transferrin-affinity method (see above), along with 25 μg of QS21 (Antigenics, New York, N.Y.) as the adjuvant. Animals were boosted twice, and hyperimmune sera were collected ca. 10 weeks from the initial immunization. For TbpA+TbpB immunizations, five mice were vaccinated with a mixture of 100 μg of affinity-purified proteins, purified via the large-scale transferrin-affinity method (see above), and Freund’s complete adjuvant. Two subsequent boosts contained 25 μg of purified protein in Freund’s incomplete adjuvant. Hyperimmune sera were collected 8 weeks postimmunization.
Immunoblotting and detection.
Western blots were probed with either the polyclonal sera described above or an antiserum raised against denatured TbpA as described previously (15). The same procedure was used to probe for gonococcal FbpA, except that the primary antibody was a rabbit serum generated against FbpA (kindly provided by Tim Meitzner). To probe for TbpB, blots were probed with horseradish peroxidase-conjugated human transferrin (Jackson Immunoresearch) and developed with ECL (Amersham).
Amino-terminal sequencing.
Affinity-purified proteins from gonococcal strain FA6905 were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a Sequi-Blot polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, Richmond, Calif.) in 10 mM CAPS [3-(cyclohexylamino)-1-propanesulfonic acid]-10% methanol (pH 11.0). Protein bands were visualized by staining with Coomassie blue R-250, and the band corresponding to the 45-kDa protein was excised. N-terminal sequencing was accomplished by automated Edman degradation on an Applied Biosystems model 477 gas phase sequencer (Midwest Analytical, Inc., St. Louis, Mo.).
RESULTS
A 45-kDa protein specifically co-affinity purified with gonococcal TbpA.
In small-scale affinity purifications from the wild-type gonococcal strain FA19 (Table 1), by Coomassie brilliant blue staining, we detected proteins with molecular masses corresponding to those of TbpA and TbpB and a smaller protein of ca. 45 kDa that either was of much lower abundance than TbpA or stained very poorly with Coomassie blue (data not shown). The same protein was also detected among the affinity-purified proteins isolated from strain FA6819, which expressed TbpA in the absence of TbpB (data not shown). In contrast, affinity purification from strain FA6747, which expressed TbpB alone, or in mock experiments that lacked transferrin, the 45-kDa protein was not detected (data not shown). These results indicated that the 45-kDa protein specifically interacted with TbpA, not with TbpB, transferrin, or the affinity matrix. Because a protein of a similar size had previously been detected in transferrin-affinity preparations and it was suggested to be FbpA (31), we probed immunoblots of the affinity-purified proteins with anti-TbpA sera, with horseradish peroxidase-conjugated human transferrin to detect TbpB, and with anti-FbpA sera. Whereas affinity preparations contained TbpA and/or TbpB as anticipated from the genotypes of the strains, none of the affinity preparations contained FbpA (data not shown), suggesting that the Tbps isolated under these conditions did not stably interact with the periplasmic binding protein.
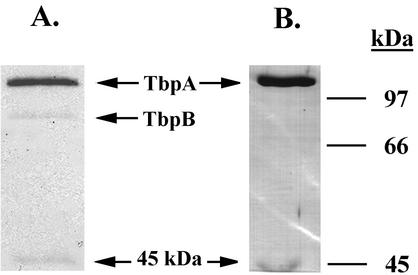
In order to generate polyclonal antisera against the Tbp complex and against TbpA, large-scale transferrin-affinity purifications were conducted by using total membrane preparations from FA19 (for the Tbp complex) and from FA6905 (for TbpA) as starting material. As with the small-scale purifications, the 45-kDa protein was isolated from strains that expressed TbpA (Fig. 1) but not from a strain that expressed TbpB alone (data not shown). The protein products represented in Fig. 1 were used as immunogens to generate polyclonal antisera as described in Materials and Methods.
FIG. 1.
Affinity purification of Tbps and the 45-kDa protein. (A) Coomassie blue-stained gel containing proteins affinity purified from gonococcal strain FA19. (B) Coomassie blue-stained gel containing proteins affinity purified from gonococcal strain FA6905. Relative positions of purified proteins are indicated between the panels; positions of molecular size standards (in kilodaltons) are indicated at the right.
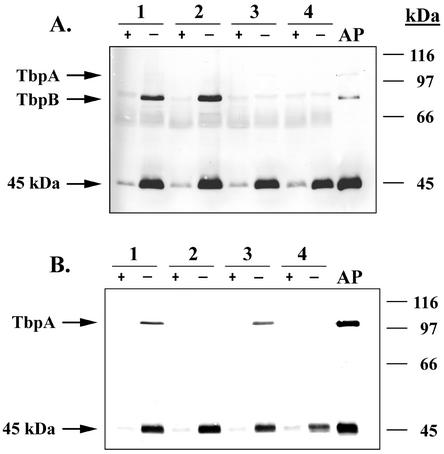
The 45-kDa protein is immunogenic and its expression is iron regulated.
Gonococcal strains FA19, FA6747, FA6905, and FA6815 (Table 1) were grown under iron-replete and iron-stressed conditions, and whole-cell proteins were subjected to SDS-PAGE and immunoblotting. The blot shown in Fig. 2A was probed with the polyclonal antisera generated against proteins purified from strain FA19; the proteins used to generate this sera are shown in Fig. 1A. The blot shown in Fig. 2B was probed with sera raised against proteins purified from strain FA6905; immunogens used to generate this sera are shown in Fig. 1B. Like TbpA and TbpB, expression of the 45-kDa protein was repressed under iron-replete conditions and induced under iron-stressed conditions (Fig. 2). The 45-kDa protein was also expressed in all strains tested, regardless of whether TbpA or TbpB was expressed. This result precluded the possibility that the 45-kDa protein was a degradation product of TbpA. Also apparent from Fig. 2 are dramatic differences in the immunogenicities of the proteins isolated by affinity purification (lanes AP in Fig. 2). Although the staining intensities of the isolated proteins decreased in the order TbpA > TbpB ≈ 45 kDa, the reactivity of the antibodies elicited was the opposite (Fig. 2A). The reactivities of both sera were the most robust against the 45-kDa protein, whereas detection of TbpA was the weakest (Fig. 2). It is possible that the immune response generated against TbpA was mainly directed toward conformational epitopes, while that elicited against TonB recognized linear epitopes that would be maintained after SDS-PAGE analysis. Overall, these results suggest a heterogeneous immune response and/or inherent differences in the immunogenicity of these proteins.
FIG. 2.
Immunogenicity and iron-regulated expression of the 45-kDa protein. (A) Lanes contain whole-cell lysates prepared from the following gonococcal strains: 1, FA19 (TbpA+ TbpB+); 2, FA6747 (TbpA− TbpB+); 3, FA6905 (TbpA+ TbpB−); and 4, FA6815 (TbpA− TbpB−). Strains were grown under iron-replete (+) and iron-stressed (−) conditions as indicated at the top and standardized by cell density. The lane labeled AP contains affinity-purified proteins that served as immunogens to generate antiserum with which the blot was probed. Molecular size standards (in kilodaltons) are indicated on the right, and the locations of relevant proteins are indicated on the left. The immunoblot was probed with polyclonal antiserum raised against affinity-purified proteins isolated from FA19. (B) Immunoblot was probed with polyclonal antiserum raised against affinity-purified proteins isolated from FA6905. Lanes are labeled as indicated for panel A.
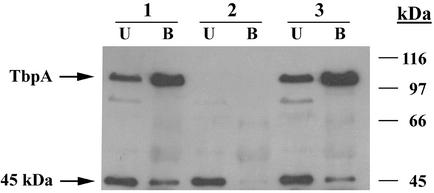
The 45-kDa protein specifically coimmunoprecipitates with gonococcal TbpA.
Antiserum raised against recombinant TbpA (45) from meningococcal strain SD (2) was used to immunoprecipitate proteins that specifically interacted with gonococcal TbpA. Because this serum also cross-reacted with gonococcal LbpA, we first generated derivatives of the wild-type and isogenic Tbp mutants, which also lacked LbpA. Total membrane preparations generated from strains MCV601, MCV602, and MCV603 (Table 1) were solubilized and immunoprecipitated with antiserum raised against recombinant TbpA. Proteins that were not precipitated with this antiserum were designated unbound (Fig. 3); proteins that were precipitated with the antiserum represented the bound fraction (Fig. 3). Bound and unbound fractions were separated by SDS-PAGE and transferred to nitrocellulose and subsequently probed with the polyclonal serum generated against proteins affinity-purified from gonococcal strain FA6905. As shown in Fig. 3, TbpA and the 45-kDa protein were coimmunoprecipitated in the TbpA+ TbpB+ strain, MCV601, as evidenced by the presence of immunoreactive proteins in the bound fraction. In order to assess the specificity of this interaction, we conducted parallel immunoprecipitation experiments by using solubilized total membrane preparations from isogenic mutants lacking either TbpA (strain MCV602) or TbpB (strain MCV603). The absence of the 45-kDa protein in the bound fraction from strain MCV602 (Fig. 3, lane 2B) and its presence in the bound fraction of strain MCV603 (Fig. 3, lane 3B) demonstrated that TbpA was required for the immunoprecipitation of the 45-kDa protein. These results are consistent with those of the affinity purifications and indicate that the 45-kDa protein specifically and stably interacts with TbpA under these experimental conditions.
FIG. 3.
Specific coimmunoprecipitation of TbpA with the 45-kDa protein. Lanes contain solubilized membrane proteins from the following strains: 1, MCV601 (TbpA+ TbpB+); 2, MCV602 (TbpA− TbpB+); and 3, MCV603 (TbpA+ TbpB−). Lanes B (bound) contain proteins that precipitated in the presence of anti-rTbpA polyclonal sera and protein A-agarose. Lanes U (unbound) contain proteins that remained in the supernatant after exposure to anti-rTbpA and protein A-agarose. The immunoblot was probed with antiserum raised against proteins affinity purified from strain FA6905. Molecular mass standards (in kilodaltons) are indicated on the right and the locations of relevant proteins are indicated on the left.
The amino terminus of the 45-kDa protein is identical to that of gonococcal TonB.
Because the 45-kDa protein was iron regulated and immunogenic and because it specifically interacted with TbpA in immunoprecipitation and affinity purification experiments, we were curious about its identity. Affinity-purified proteins from strain FA6905 (Fig. 1B) were separated by SDS-PAGE and transferred to PVDF. The resulting blot was stained with Coomassie blue, and the 45-kDa band was excised and subjected to sequential Edman degradation. From this analysis we determined that the N-terminal sequence of the 45-kDa band (MDKERILTPAVV) was identical to the amino terminus of gonococcal TonB (7).
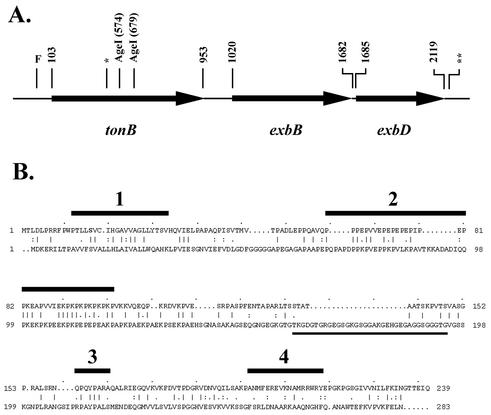
An internal deletion mutant of gonococcal TonB was capable of transferrin-iron utilization.
Using primers derived from the published sequence of gonococcal tonB (7), the entire gene, including a 10-bp gonococcal uptake sequence (Fig. 4A), was amplified by PCR and cloned. The resulting plasmid, pVCU689 (Table 1), was mutagenized by deletion of a 105-bp AgeI fragment (Fig. 4A), resulting in an in-frame deletion within the TonB-encoding open reading frame (Fig. 4B). The mutagenized plasmid, pVCU690 (Table 1), was subsequently transformed into gonococcal strain FA19, resulting in strain MCV607 (Table 1). We also cloned an Ω interposon encoding gentamicin resistance (3) into the deleted tonB gene, generating plasmid pVCU691 (Table 1), which was transformed into gonococcal strain MCV601, selecting for gentamicin resistance. This polar insertion into tonB generated a knockout of the entire operon, since the genes encoding the other members of the complex, exbB and exbD, are located immediately downstream of tonB (Fig. 4A) (7). The resulting TonB mutant, MCV609 (Table 1), was grown under iron-stressed conditions, along with strains MCV601, MCV607, and MCV604, an Lbp− derivative of a previously described TonB-box mutant of TbpA (13). Whole-cell lysates generated from these strains were probed with antiserum that recognized TbpA and TonB (Fig. 5A). As anticipated, MCV601 and MCV604 expressed wild-type-sized TbpA and TonB (Fig. 5A, lanes 1 and 2). The in-frame deletion mutant, MCV607, expressed a significantly smaller version of TonB, and MCV609 expressed no detectable TonB at all (Fig. 5A, lanes 3 and 4). We analyzed the ability of these Ton system mutants to grow on transferrin as a sole iron source in comparison with control strains. While the TbpA− mutant failed to grow, the in-frame deletion mutant of TonB surprisingly supported the growth of the gonococcus on transferrin-iron (data not shown). The TonB knockout strain, MCV609, failed to grow on transferrin (data not shown), a finding consistent with its inability to express the energy transduction system.
FIG. 4.
Genetic organization of the gonococcal Ton system operon and amino acid sequence alignment between E. coli and gonococcal TonB proteins. (A) Genes encoding the components of the gonococcal Ton system, including the location of unique AgeI restriction sites within tonB. Bases are numbered according to the sequence submitted to GenBank under the accession number U79563. The approximate position of a 10-bp gonococcal uptake sequence within tonB is indicated by a single asterisk; the previously characterized gonococcal uptake sequence at the 3′ terminus of exbD (7) is indicated by the double asterisk. F indicates the approximate position of the Fur box that lies upstream of the tonB gene. (B) Pairwise amino acid sequence alignment between E. coli TonB (top) and gonococcal TonB (bottom). The E. coli sequence is shown as being initiated at the third possible Met (37), with amino acids numbered consecutively from that position. The four regions identified by homology and function in enterobacterial TonB proteins (28) are indicated by the numbered (1 to 4) horizontal black bars above the E. coli sequence. Region 1 corresponds to the transmembrane domain; region 2 contains the proline-rich region; region 3 contains the Q160 region and downstream conserved residues; region 4 contains an interaction motif predicted to form an amphipathic helix (28). The region that was deleted from gonococcal TonB in the present study is underlined.
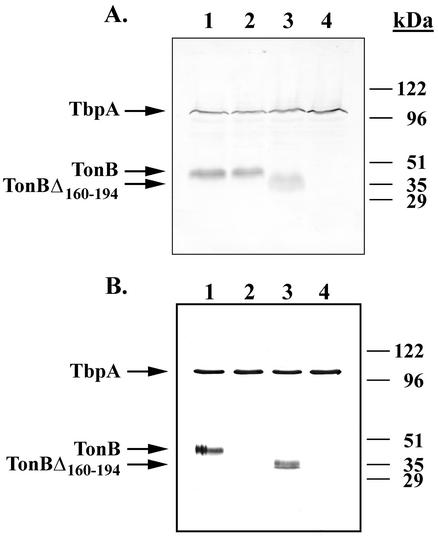
FIG. 5.
Characterization of TonB mutants. (A) Lanes contain whole-cell lysates generated from the following strains: 1, MCV601 (TbpA+ TbpB+); 2, MCV604 (TbpA TonB box− TbpB+); 3, MCV607 (TbpA+ TbpB+, TonBΔ160-194); and 4, MCV609 (TbpA+ B+, tonB::Ω). The immunoblot was probed with antiserum raised against proteins affinity purified from strain FA6905. (B) Lanes contain proteins transferrin-affinity purified from the following strains: 1, MCV601 (TbpA+ TbpB+); 2, MCV604 (TbpA TonB box− TbpB+); 3, MCV607 (TbpA+ TbpB+, TonBΔ160-194); and 4, MCV609 (TbpA+ TbpB+, tonB::Ω). The immunoblot was probed with antiserum raised against proteins affinity purified from strain FA6905.
The TonB deletion mutant was capable of stable interaction with TbpA, although a TbpA TonB-box mutant did not interact with TonB.
We previously demonstrated that a single residue alteration in the TonB box of TbpA, converting Ile at position 16 to Pro, is sufficient to completely inactivate the gonococcal transferrin-iron acquisition system (13). This mutation rendered the gonococcus unable to grow on transferrin-bound iron; however, transferrin binding by the mutant receptor was actually increased, possibly due to impairment of ligand release (13). From our previous analysis, it was unclear whether TonB interacted with the mutant TonB box of TbpA or if the results of the interaction were simply nonproductive. Thus, we analyzed the ability of the TbpA TonB-box mutant to facilitate copurification of TbpA and TonB in a transferrin-affinity purification assay. As shown in Fig. 5B, TonB was not identified in affinity purifications from strain MCV604 (Fig. 5B, lane 2), indicating that a productive and stable interaction could not be maintained between TonB and the mutated TbpA. Surprisingly, a deletion mutation of TonB, which removed 35 amino acids from the central portion of the protein, did not impair the interaction between TonB and TbpA (Fig. 5B, lane 3), a finding consistent with the apparently wild-type function of this protein.
DISCUSSION
Although the TbpA component of the gonococcal transferrin receptor shares many characteristics with TonB-dependent siderophore transporters, distinct differences are also apparent. In contrast to siderophore receptors, TbpA acts in concert with a lipoprotein, TbpB, to efficiently acquire transferrin-bound iron (4). The addition of this second protein component, with which TbpA is likely complexed (8, 13, 16; unpublished observations), may affect the kinetics and mechanics of the energy transduction process. Another major difference between siderophore transporters and TbpA is that the process of iron acquisition from transferrin requires an iron removal event at the cell surface. Ferric siderophores cross the outer membrane intact, whereas transferrin is deferrated at the surface, with subsequent internalization of iron alone (42). Although it is clear that TonB-derived energy is necessary for the release of transferrin from the receptor (13), the initial energy-requiring step may be the iron-stripping event at the cell surface, which is potentially a prerequisite for release of the deferrated ligand. Another reflection of the divergence between the energy transduction processes of the neisseriae versus E. coli comes from the identification of little sequence conservation between the TonB proteins of these species (44). Given the divergence, it is perhaps not surprising that meningococcal TonB could not support TonB-dependent transport of ferrichrome by FhuA in E. coli (44) and that E. coli TonB could not support transferrin-iron internalization by gonococcal TbpA (14).
In the present study, we used affinity chromatography to isolate transferrin-binding proteins from N. gonorrhoeae and the resulting samples were used as immunogens for the development of polyclonal antisera. Analysis of the affinity-purified samples indicated that the preparations were not completely devoid of additional proteins. A 45-kDa protein was also affinity purified, but only from strains that expressed TbpA. Contamination of affinity-purified samples by low-molecular-weight species has been noted previously (40) in affinity isolation of the lactoferrin receptor from N. meningitidis. Later attempts to compare Tbps and Lbps among different gonococcal isolates identified a ca. 37-kDa species, which was suggested to be FbpA (31), the periplasmic, ferric binding protein (1).
The copurifying protein detected in the present study was unambiguously identified as gonococcal TonB. However, in contrast to its predicted molecular mass of 28 kDa (7), the species that copurified with TbpA under the current conditions migrated in SDS-PAGE at an apparent molecular mass of 45 kDa. This is consistent with observations of E. coli TonB, which has a predicted molecular mass of 25 kDa but a relative mobility corresponding to ca. 35 kDa. A recent study (11) describing the crystal structure of a C-terminal domain of E. coli TonB suggested that TonB might exist as a dimer. Chang et al. identified a novel structural motif in which three β-strands of one monomer are woven together with those of another monomer to form a large antiparallel β-sheet. However, in another recent study, Moeck and Letellier (34) determined that E. coli TonB formed mostly monomers in solution, although that study confirmed the aberrant migration of TonB in polyacrylamide gels. Both studies indicated that TonB existed as an elongated structure that might contribute to its unexpected migration pattern. TonB is also one of the most proline-rich proteins known in nature (47), which likewise contributes to its unusual biochemical characteristics. Deletion of the proline-rich motif from E. coli TonB resulted in a protein with a relative mobility consistent with the sequence-predicted molecular mass, indicating that this motif is entirely responsible for the aberrant migration of wild-type TonB (30).
Another consequence of the unusual sequence of TonB could be the immunodominant immune response generated against this protein, relative to the other proteins with which it was copurified. Although the amount of TonB co-affinity purified with TbpA was apparently low, as evaluated by Coomassie blue staining, leading us to initially conclude that it represented a minor contaminant, the immune response generated against this protein was quite robust. The relatively high immunogenicity of TonB, in comparison to that of the transferrin-binding proteins, may have implications for vaccine studies that evaluate the immune response to Tbps affinity-purified directly from the pathogenic Neisseria species. The immune response to the transferrin-binding proteins could be overestimated due to the unanticipated presence of TonB in antigen preparations.
The polyvalent sera generated in the present study allowed us to evaluate the expression of TonB as a function of iron stress. Like the Tbps, TonB expression was dramatically influenced by iron availability, prompting us to search the gonococcal tonB locus (7) for evidence of a Fur binding site. Stojiljkovic et al. (44) identified a potential Fur binding site 40 bp upstream of the 5′ end of meningococcal tonB. Similarly, we identified an identical sequence 36 bp upstream from the start codon of tonB in gonococcal strain FA19, a finding consistent with our observation of iron-repressed expression. Although we did not attempt to quantitate the iron-dependent repression of TonB expression, it is clear that neisserial TonB is induced under iron-stressed conditions. This is consistent with observations of E. coli TonB, which is repressed threefold by growth under high-iron conditions and is additionally regulated by anaerobiosis (48).
This study allowed us to characterize the requirements for and specificity of the association between TbpA and TonB. We consistently demonstrated the presence of TonB in transferrin-affinity purifications only from strains that expressed TbpA. The presence of TbpB was not required nor was it sufficient for copurification of TonB. Addition of exogenous cross-linkers was also not necessary to demonstrate a TbpA-TonB interaction, suggesting that the association of these proteins was quite stable. Since both affinity purification and immunoprecipitation procedures utilize solubilized membrane proteins as starting material, we cannot definitively determine whether interaction between TbpA and TonB occurred in vivo and withstood solubilization or if stable association between these proteins occurred in vitro subsequent to solubilization. Because the immunoprecipitations were conducted in the absence of added transferrin, the observation of TbpA-TonB complexes in this assay suggests that ligand binding is not a prerequisite for TonB association; however, we did not directly evaluate the concentration dependence of this interaction in the present study.
Using Ton system mutants, we probed the requirements for TbpA-TonB interaction. A point mutation converting a hydrophobic amino acid (Ile) to a turn-promoting residue (Pro) in the TonB box of TbpA was sufficient to eliminate interaction between these proteins. This is consistent with the results of our previous study, which indicated that this TonB-box mutant was incapable of transferrin-iron transport (13). In addition, Larsen et al. found that similar TonB-box mutations in E. coli FepA prevented in vivo chemical cross-linking between TonB and FepA (27). Recent studies by Cadieux et al. (9), Cadieux and Kadner (10), and Coggshall et al. (12) have demonstrated that similar transport-defective mutants in the BtuB TonB box likewise have dramatic effects on the ability of TonB to interact with and cross-link to BtuB. These authors suggested that the conformation of the region consisting of the TonB box is altered by such mutations, thus preventing a productive interaction with and energy-coupling by TonB. It has been suggested that other sites of receptor-TonB interaction outside the defined TonB box are likely (10, 25, 27, 41). Mutant FhuA (25) and FepA (41) proteins completely lacking the plug domain and TonB box retain TonB-dependent transport activities, suggesting that while interactions between TonB and the TonB box are required for efficient transport, other barrel-specific interactions also play a role in substrate internalization. The present study suggests that either such barrel-specific interactions are too unstable to facilitate copurification after TonB-box interactions are lost, or that interaction via the TonB box is a necessary prerequisite for subsequent interactions. It is also conceivable that the altered conformation of the mutant TonB box physically interferes with subsequent or simultaneous barrel interactions, whereas complete removal of the plug and the TonB box did not result in the complete elimination of energy-dependent functions.
In contrast to the TonB-box mutant, the loss of 35 amino acids within the TonB protein did not ablate association with the TonB-dependent receptor. This in-frame deletion mutant, resulting from the loss of residues 160 to 194, remained competent for interaction with TbpA and mediated energization of the receptor such that iron internalization was afforded. The deleted amino acids fell within a Neisseria-specific region rich in glycine residues but outside of previously recognized domains shared by TonB proteins from the family Enterobacteriaceae (28), one of which consists of Pro and Lys residues. Larsen et al. (30) conducted a deletion analysis of E. coli TonB, from which it was concluded that residues 66 to 100, consisting of the repeat motif (Glu-Pro)n-(Lys-Pro)m, were not required for energy transduction but likely played a role in extension of the protein across the periplasmic space. Conversely, FepA-TonB interaction was shown to depend on the carboxy-terminal region of TonB (27, 32), which includes the fourth region of homology and a putative amphipathic helix motif (28) identified in the enterobacterial TonB proteins. The deletion constructed in the present study removed 35 amino acids of gonococcal TonB that were located between these two conserved domains in the homologous E. coli TonB sequence. The transport-competent phenotype of this mutant is consistent with the suggestion that the central portion of the protein is not required for its energy transduction and receptor interaction functions.
In conclusion, we identified a protein with an apparent molecular mass of 45 kDa that specifically copurified with TbpA. Amino-terminal sequencing of this copurified protein identified it as gonococcal TonB. In addition to interaction with TbpA in transferrin-affinity preparations, we demonstrated a TonB-TbpA interaction in the absence of added ligand in immunoprecipitation experiments. Moreover, we demonstrated that expression of gonococcal TonB was strongly influenced by iron availability and that TonB was very immunogenic in experimental animals. The TonB-TbpA complex could be isolated in the absence of chemical cross-linkers and after deletion of 35 amino acids internal to the TonB protein. In contrast, a TbpA point mutation in the characterized TonB box resulted in elimination of complex formation by TonB and TbpA, which dramatically demonstrated that physical interaction between TonB and a TonB-dependent receptor depended on a wild-type sequence near the amino terminus of the receptor. Thus, characterization of what seemed to be a minor contaminant of TbpA purifications led to further insights into this intriguing energy-transducing protein.
Acknowledgments
This work was supported by Public Health Service grants AI39523 and AI47141 from the National Institute of Allergy and Infectious Diseases.
We are grateful for the contributions of antisera from Andrew Gorringe (against recombinant meningococcal TbpA) and Tim Meitzner (against gonococcal FbpA). We also thank Gour Biswas and Fred Sparling for providing chromosomal DNA from gonococcal strain FA6839 and acknowledge the assistance of Thomas Metcalf of Wyeth Research in the generation of anti-TbpA sera. The TbpA+TbpB sera were generated at Covance Research Products, Inc. We also acknowledge Dave McCourt of Midwest Analytical, Inc., St. Louis, Mo., for performing the amino-terminal protein sequencing and Mikhail F. Alexeyev for providing plasmid pBSL142, the source of the Ω gentamicin cassette. We also gratefully acknowledge Kathleen Postle for critical reading of the manuscript and for providing insightful comments.
REFERENCES
- 1.Adhikari, P., S. A. Berish, A. J. Nowalk, K. L. Veraldi, S. A. Morse, and T. A. Mietzner. 1996. The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J. Bacteriol. 178:2145-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ala'Aldeen, D. A. A., P. Stevenson, E. Griffiths, A. R. Gorringe, L. I. Irons, A. Robinson, S. Hyde, and S. P. Borriello. 1994. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect. Immun. 62:2984-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexeyev, M. F., I. N. Shokolenko, and T. P. Croughan. 1995. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene 160:63-67. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, J. E., P. F. Sparling, and C. N. Cornelissen. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 176:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, P. E., C. D. Nau, J. T. Brown, J. Konisky, and R. J. Kadner. 1990. Genetic suppression demonstrates interaction of TonB protein with outer membrane transport proteins in Escherichia coli. J. Bacteriol. 172:3826-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas, G. D., J. E. Anderson, C.-J. Chen, C. N. Cornelissen, and P. F. Sparling. 1999. Identification and functional characterization of the Neisseria gonorrhoeae lbpB gene product. Infect. Immun. 67:455-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas, G. D., J. E. Anderson, and P. F. Sparling. 1997. Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB, and exbD genes. Mol. Microbiol. 24:169-179. [DOI] [PubMed] [Google Scholar]
- 8.Boulton, I. C., A. R. Gorringe, R. J. R. Carr, B. Gorinsky, C. L. Joannou, and R. W. Evans. 1997. Characterization of the meningococcal transferrin binding protein complex by photon correlation spectroscopy. FEBS Lett. 414:409-413. [DOI] [PubMed] [Google Scholar]
- 9.Cadieux, N., C. Bradbeer, and R. J. Kadner. 2000. Sequence changes in the Ton box of BtuB affect its transport activities and interaction with TonB protein. J. Bacteriol. 182:5954-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadieux, N., and R. J. Kadner. 1999. Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc. Natl. Acad. Sci. USA 96:10673-10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, C., A. Mooser, A. Pluckthun, and A. Wlodawer. 2001. Crystal structure of the dimeric C-terminal domain of TonB reveals a novel fold. J. Biol. Chem. 276:27535-27540. [DOI] [PubMed] [Google Scholar]
- 12.Coggshall, K. A., N. Cadieux, C. Piedmont, R. J. Kadner, and D. S. Cafiso. 2001. Transport-defective mutations alter the conformation of the energy-coupling motif of the outer membrane transporter. Biochemistry 40:13964-13971. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen, C. N., J. E. Anderson, and P. F. Sparling. 1997. Energy-dependent changes in the gonococcal transferrin receptor. Mol. Microbiol. 26:25-35. [DOI] [PubMed] [Google Scholar]
- 14.Cornelissen, C. N., G. D. Biswas, and P. F. Sparling. 1993. Expression of gonococcal transferrin-binding protein 1 causes Escherichia coli to bind human transferrin. J. Bacteriol. 175:2448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelissen, C. N., G. D. Biswas, J. Tsai, D. K. Paruchuri, S. A. Thompson, and P. F. Sparling. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 174:5788-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelissen, C. N., and P. F. Sparling. 1996. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J. Bacteriol. 178:1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelissen, C. N., and P. F. Sparling. 1994. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol. Microbiol. 14:843-850. [DOI] [PubMed] [Google Scholar]
- 18.Gudmundsdottir, A., P. E. Bell, M. D. Lundrigan, C. Bradbeer, and R. J. Kadner. 1989. Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J. Bacteriol. 171:6526-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunter, K., and V. Braun. 1990. In vivo evidence for FhuA outer membrane receptor interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 274:85-88. [DOI] [PubMed] [Google Scholar]
- 20.Heller, K. J., R. J. Kadner, and K. Gunther. 1988. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene 64:147-153. [DOI] [PubMed] [Google Scholar]
- 21.Higgs, P. I., P. S. Myers, and K. Postle. 1998. Interactions in the TonB-dependent energy transduction complex: ExbB and ExbD form homomultimers. J. Bacteriol. 180:6031-6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaskula, J. C., T. E. Letain, S. K. Roof, J. T. Skare, and K. Postle. 1994. Role of the TonB amino terminus in energy transduction between membranes. J. Bacteriol. 176:2326-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsson, M., K. Hannavy, and C. F. Higgins. 1993. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol. Microbiol. 8:379-388. [DOI] [PubMed] [Google Scholar]
- 24.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Killmann, H., M. Braun, C. Herrmann, and V. Braun. 2001. FhuA barrel-cork hybrids are active transporters and receptors. J. Bacteriol. 183:3476-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Larsen, R. A., D. Foster-Hartnett, M. A. McIntosh, and K. Postle. 1997. Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J. Bacteriol. 179:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen, R. A., P. S. Myers, J. T. Skare, C. L. Seachord, R. P. Darveau, and K. Postle. 1996. Identification of TonB homologs in the family Enterobacteriaceae and evidence for conservation of TonB-dependent energy transduction complexes. J. Bacteriol. 178:1363-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen, R. A., M. G. Thomas, and K. Postle. 1999. Protonmotive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol. Microbiol. 31:1809-1824. [DOI] [PubMed] [Google Scholar]
- 30.Larsen, R. A., G. E. Wood, and K. Postle. 1993. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol. Microbiol. 10:943-953. [DOI] [PubMed] [Google Scholar]
- 31.Lee, B. C., and L. E. Bryan. 1989. Identification and comparative analysis of the lactoferrin and transferrin receptors among clinical isolates of gonococci. J. Med. Microbiol. 28:199-204. [DOI] [PubMed] [Google Scholar]
- 32.Letain, T. E., and K. Postle. 1997. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in E. coli. Mol. Microbiol. 24:271-283. [DOI] [PubMed] [Google Scholar]
- 33.Mickelsen, P. A., and P. F. Sparling. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moeck, G. S., and L. Letellier. 2001. Characterization of in vitro interactions between a truncated TonB protein from Escherichia coli and the outer membrane receptors FhuA and FepA. J. Bacteriol. 183:2755-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 36.Postle, K. 2002. Close before opening. Science 295:1658-1659. [DOI] [PubMed] [Google Scholar]
- 37.Postle, K., and J. T. Skare. 1988. Escherichia coli TonB protein is exported from the cytoplasm without proteolytic cleavage of its amino terminus. J. Biol. Chem. 263:11000-11007. [PubMed] [Google Scholar]
- 38.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 39.Schoffler, H., and V. Braun. 1989. Transport across the outer membrane of Escherichia coli K-12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol. Gen. Genet. 217:378-383. [DOI] [PubMed] [Google Scholar]
- 40.Schryvers, A. B., and L. J. Morris. 1988. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect. Immun. 56:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott, D. C., Z. Cao, Z. Qi, M. Bauler, J. D. Igo, S. M. C. Newton, and P. E. Klebba. 2001. Exchangeability of N termini in the ligand gated porins of Escherichia coli. J. Biol. Chem. 276:13025-13033. [DOI] [PubMed] [Google Scholar]
- 42.Simonson, C., D. Brener, and I. W. DeVoe. 1982. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect. Immun. 36:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skare, J. T., B. M. M. Ahmer, C. L. Seachord, R. P. Darveau, and K. Postle. 1993. Energy transduction between membranes: TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J. Biol. Chem. 268:16302-16308. [PubMed] [Google Scholar]
- 44.Stojiljkovic, I., and N. Srinivasan. 1997. Neisseria meningitidis tonB, exbB, and exbD genes: Ton-dependent utilization of protein-bound iron in neisseriae. J. Bacteriol. 179:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West, D., K. Reddin, M. Matheson, R. Heath, S. Funnell, M. Hudson, A. Robinson, and A. Gorringe. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect. Immun. 69:1561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West, S. E. H., and P. F. Sparling. 1987. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J. Bacteriol. 169:3414-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson, M. P. 1994. The structure and function of proline-rich proteins. Biochem. J. 297:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young, G. M., and K. Postle. 1994. Repression of tonB transcription during anaerobic growth requires Fur binding at the promoter and a second factor binding upstream. Mol. Microbiol. 11:943-954. [DOI] [PubMed] [Google Scholar]