Abstract
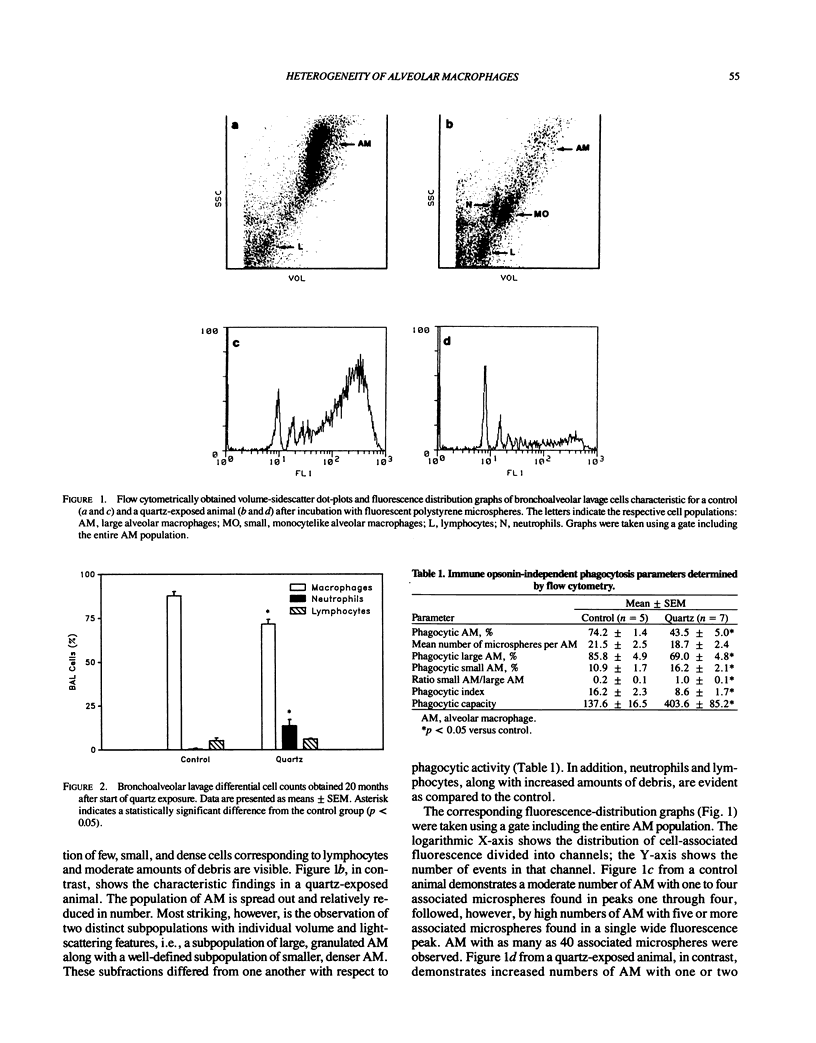
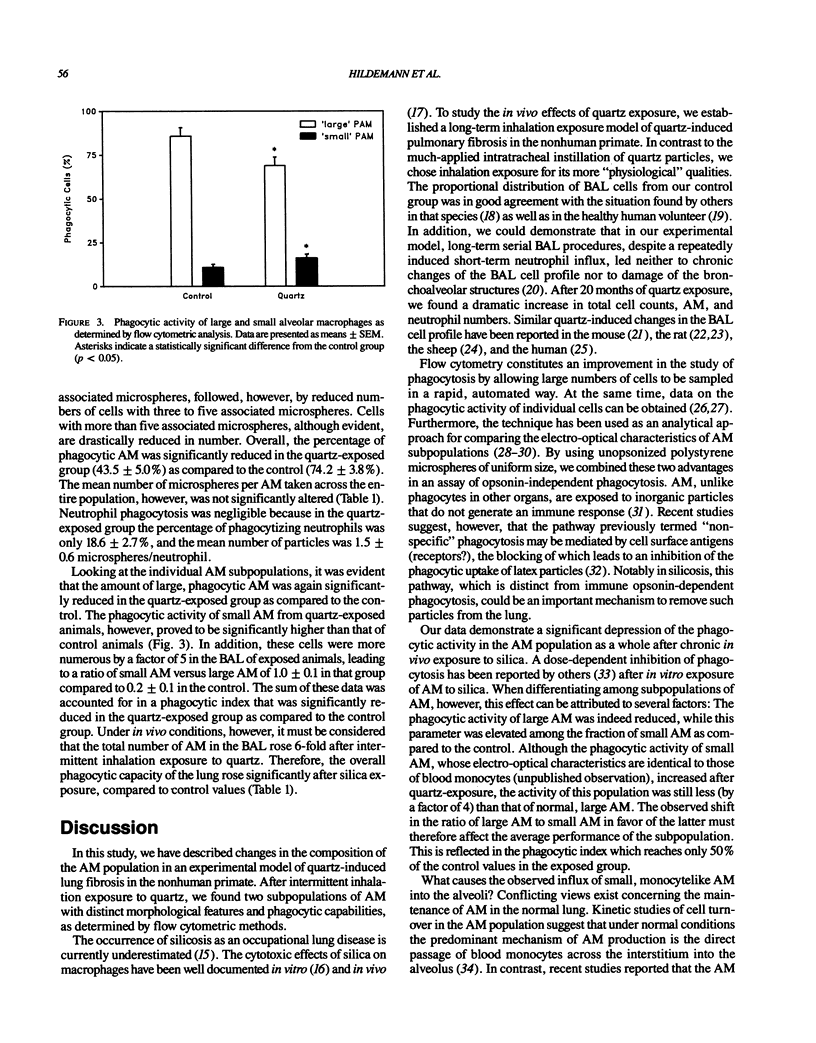
The alveolar macrophage (AM) population has been shown to be heterogeneous in composition as well as in function. The aim of our study was to assess morphological and functional features of AM in an experimental model of quartz-induced lung fibrosis by flow cytometric methods. Twelve cynomolgus monkeys were exposed 8 hr/day, 5 days/week for 26 months to either normal atmosphere (n = 5) or 5 mg/m3 DQ12 less than 5 microns quartz dust (n = 7). After 20 months of exposure, we studied AM phagocytosis by incubating bronchoalveolar lavage cells with fluorescent polystyrene microspheres (mean diameter 1.91 microns). Using a fluorescence-activated cell sorter analyzer, AM subpopulations were identified via their volume/side scatter properties. After selective electronic "gating" of the AM populations, both the percentage of phagocytic AM and the mean number of ingested microspheres per AM were determined. In addition, a phagocytic index (microspheres/AM x % phagocytic AM x 10(-2) and a hypothetical total phagocytic capacity of one lung (phagocytic index x total number of AM x 10(-6) were calculated. The total bronchoalveolar lavage cell counts rose (75.6 +/- 11.3 x 10(6) versus 10.1 +/- 0.8 x 10(6)) significantly after quartz exposure. In contrast, the percentage of phagocytic AM was significantly (p less than 0.05) reduced (43.5 +/- 5.0% versus 74.2 +/- 1.4%). Flow cytometric measurements revealed the appearance of an AM subpopulation characterized by size/granularity features identical to blood monocytes. Only minimal numbers of these cells were found under normal conditions, but they constituted 50% of the entire AM population in the quartz group.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Role of monocytes and interstitial cells in the generation of alveolar macrophages II. Kinetic studies after carbon loading. Lab Invest. 1980 May;42(5):518–524. [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Role of monocytes and interstitial cells in the generation of alveolar macrophages I. Kinetic studies of normal mice. Lab Invest. 1980 May;42(5):511–517. [PubMed] [Google Scholar]
- Brown G. P., Monick M., Hunninghake G. W. Fibroblast proliferation induced by silica-exposed human alveolar macrophages. Am Rev Respir Dis. 1988 Jul;138(1):85–89. doi: 10.1164/ajrccm/138.1.85. [DOI] [PubMed] [Google Scholar]
- Bégin R. O., Cantin A. M., Boileau R. D., Bisson G. Y. Spectrum of alveolitis in quartz-exposed human subjects. Chest. 1987 Dec;92(6):1061–1067. doi: 10.1378/chest.92.6.1061. [DOI] [PubMed] [Google Scholar]
- Bégin R., Dufresne A., Cantin A., Possmayer F., Sébastien P., Fabi D., Bilodeau G., Martel M., Bisson D., Pietrowski B. Quartz exposure, retention, and early silicosis in sheep. Exp Lung Res. 1989 May;15(3):409–428. doi: 10.3109/01902148909087868. [DOI] [PubMed] [Google Scholar]
- Calhoun W. J., Salisbury S. M. Heterogeneity in cell recovery and superoxide production in buoyant, density-defined subpopulations of human alveolar macrophages from healthy volunteers and sarcoidosis patients. J Lab Clin Med. 1989 Dec;114(6):682–690. [PubMed] [Google Scholar]
- Callis A. H., Sohnle P. G., Mandel G. S., Wiessner J., Mandel N. S. Kinetics of inflammatory and fibrotic pulmonary changes in a murine model of silicosis. J Lab Clin Med. 1985 May;105(5):547–553. [PubMed] [Google Scholar]
- Chandler D. B., Fuller W. C., Jackson R. M., Fulmer J. D. Studies of membrane receptors and phagocytosis in subpopulations of rat alveolar macrophages. Am Rev Respir Dis. 1986 Mar;133(3):461–467. doi: 10.1164/arrd.1986.133.3.461. [DOI] [PubMed] [Google Scholar]
- Davis G. S. Pathogenesis of silicosis: current concepts and hypotheses. Lung. 1986;164(3):139–154. doi: 10.1007/BF02713638. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Lehnert B. E. Pulmonary interstitial macrophages: isolation and flow cytometric comparisons with alveolar macrophages and blood monocytes. J Leukoc Biol. 1988 Jan;43(1):80–90. doi: 10.1002/jlb.43.1.80. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Lindenschmidt R. C., Maurer J. K., Higgins J. M., Ridder G. Pulmonary response to silica or titanium dioxide: inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am J Respir Cell Mol Biol. 1990 Apr;2(4):381–390. doi: 10.1165/ajrcmb/2.4.381. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Feltner D. E., Brieland J. K., Ward P. A. Phagocytic cell-derived inflammatory mediators and lung disease. Chest. 1987 Mar;91(3):428–435. doi: 10.1378/chest.91.3.428. [DOI] [PubMed] [Google Scholar]
- Gant V. A., Hamblin A. S. Human bronchoalveolar macrophage heterogeneity demonstrated by histochemistry, surface markers and phagocytosis. Clin Exp Immunol. 1985 Jun;60(3):539–545. [PMC free article] [PubMed] [Google Scholar]
- Haley P. J., Muggenburg B. A., Rebar A. H., Shopp G. M., Bice D. E. Bronchoalveolar lavage cytology in cynomolgus monkeys and identification of cytologic alterations following sequential saline lavage. Vet Pathol. 1989 May;26(3):265–273. doi: 10.1177/030098588902600312. [DOI] [PubMed] [Google Scholar]
- Kelley J. Cytokines of the lung. Am Rev Respir Dis. 1990 Mar;141(3):765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- Kradin R. L., McCarthy K. M., Preffer F. I., Schneeberger E. E. Flow-cytometric and ultrastructural analysis of alveolar macrophage maturation. J Leukoc Biol. 1986 Oct;40(4):407–417. doi: 10.1002/jlb.40.4.407. [DOI] [PubMed] [Google Scholar]
- Lehnert B. E., Valdez Y. E., Fillak D. A., Steinkamp J. A., Stewart C. C. Flow cytometric characterization of alveolar macrophages. J Leukoc Biol. 1986 Mar;39(3):285–298. doi: 10.1002/jlb.39.3.285. [DOI] [PubMed] [Google Scholar]
- Parod R. J., Brain J. D. Immune opsonin-independent phagocytosis by pulmonary macrophages. J Immunol. 1986 Mar 15;136(6):2041–2047. [PubMed] [Google Scholar]
- Parod R. J., Brain J. D. Uptake of latex particles by macrophages: characterization using flow cytometry. Am J Physiol. 1983 Sep;245(3):C220–C226. doi: 10.1152/ajpcell.1983.245.3.C220. [DOI] [PubMed] [Google Scholar]
- Parod R. J., Godleski J. J., Brain J. D. Inhibition of immune opsonin-independent phagocytosis by antibody to a pulmonary macrophage cell surface antigen. J Immunol. 1986 Mar 15;136(6):2048–2054. [PubMed] [Google Scholar]
- Robock K. Standard quartz dq12 greater than 5 micro m for experimental pneumoconiosis research projects in the Federal Republic of Germany. Ann Occup Hyg. 1973 Apr;16(1):63–66. doi: 10.1093/annhyg/16.1.63. [DOI] [PubMed] [Google Scholar]
- Shellito J., Esparza C., Armstrong C. Maintenance of the normal rat alveolar macrophage cell population. The roles of monocyte influx and alveolar macrophage proliferation in situ. Am Rev Respir Dis. 1987 Jan;135(1):78–82. doi: 10.1164/arrd.1987.135.1.78. [DOI] [PubMed] [Google Scholar]
- Shellito J., Kaltreider H. B. Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages. II. Activation as a determinant of functional activity. Am Rev Respir Dis. 1985 May;131(5):678–683. doi: 10.1164/arrd.1985.131.5.678. [DOI] [PubMed] [Google Scholar]
- Steinkamp J. A., Wilson J. S., Saunders G. C., Stewart C. C. Phagocytosis: flow cytometric quantitation with fluorescent microspheres. Science. 1982 Jan 1;215(4528):64–66. doi: 10.1126/science.7053559. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Lehnert B. E., Steinkamp J. A. In vitro and in vivo measurement of phagocytosis by flow cytometry. Methods Enzymol. 1986;132:183–192. doi: 10.1016/s0076-6879(86)32006-8. [DOI] [PubMed] [Google Scholar]
- Struhar D., Harbeck R. J., Mason R. J. Lymphocyte populations in lung tissue, bronchoalveolar lavage fluid, and peripheral blood in rats at various times during the development of silicosis. Am Rev Respir Dis. 1989 Jan;139(1):28–32. doi: 10.1164/ajrccm/139.1.28. [DOI] [PubMed] [Google Scholar]
- Takemura T., Rom W. N., Ferrans V. J., Crystal R. G. Morphologic characterization of alveolar macrophages from subjects with occupational exposure to inorganic particles. Am Rev Respir Dis. 1989 Dec;140(6):1674–1685. doi: 10.1164/ajrccm/140.6.1674. [DOI] [PubMed] [Google Scholar]
- Valiante D. J., Rosenman K. D. Does silicosis still occur? JAMA. 1989 Dec 1;262(21):3003–3007. [PubMed] [Google Scholar]
- Zimmerman B. T., Canono B. P., Campbell P. A. Silica decreases phagocytosis and bactericidal activity of both macrophages and neutrophils in vitro. Immunology. 1986 Dec;59(4):521–525. [PMC free article] [PubMed] [Google Scholar]


