Abstract
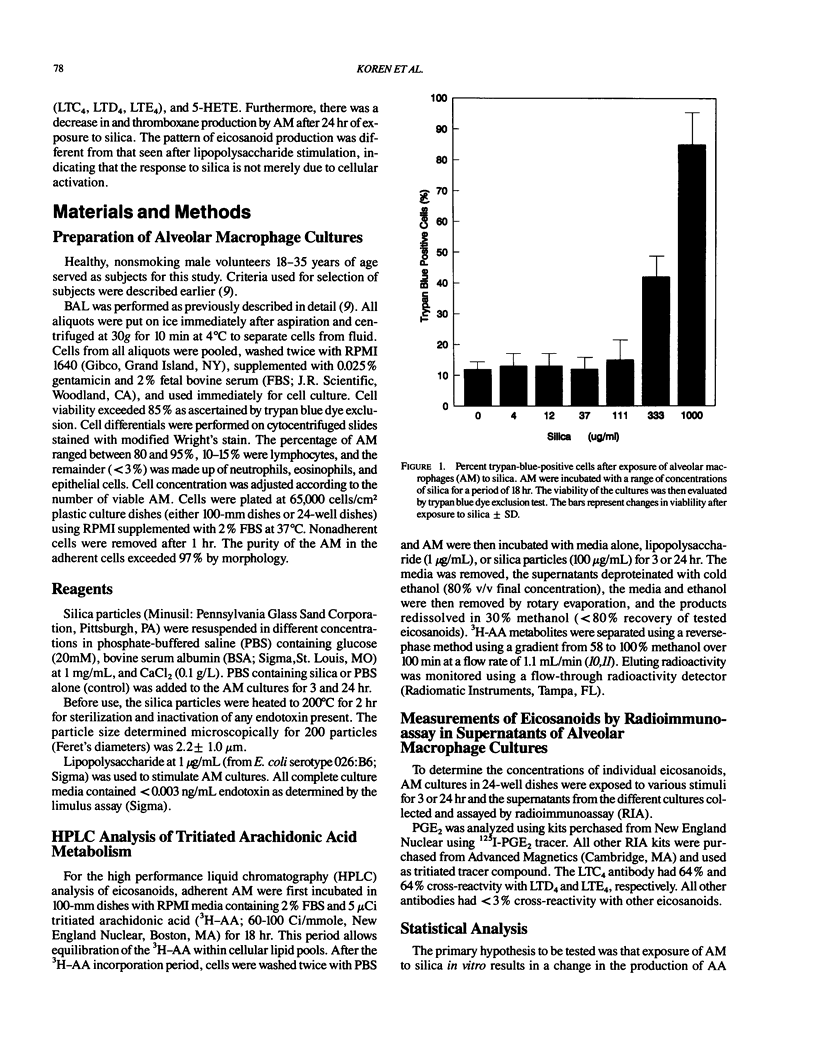
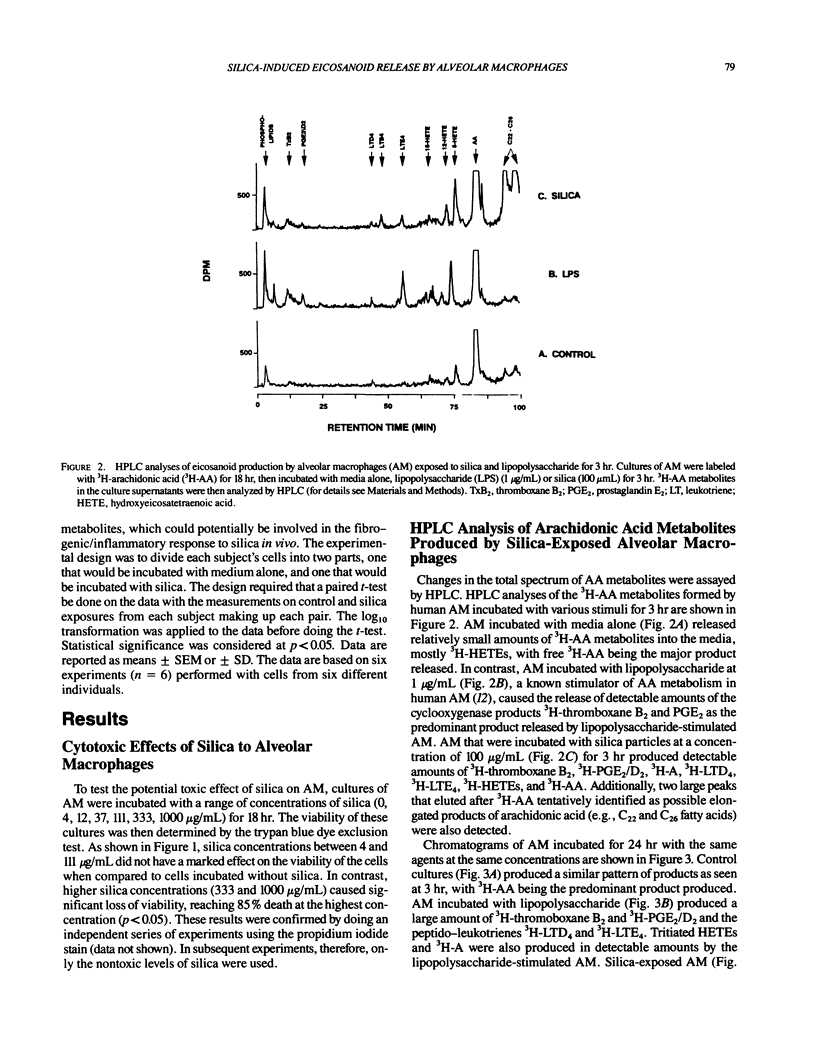
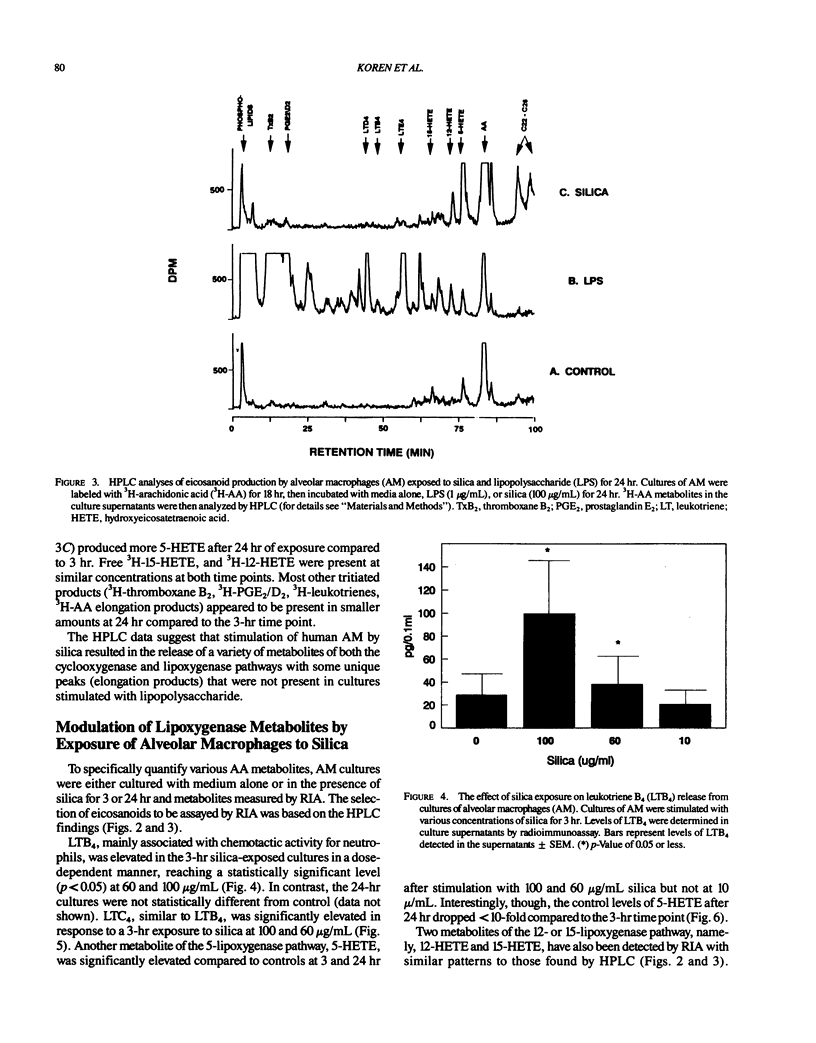
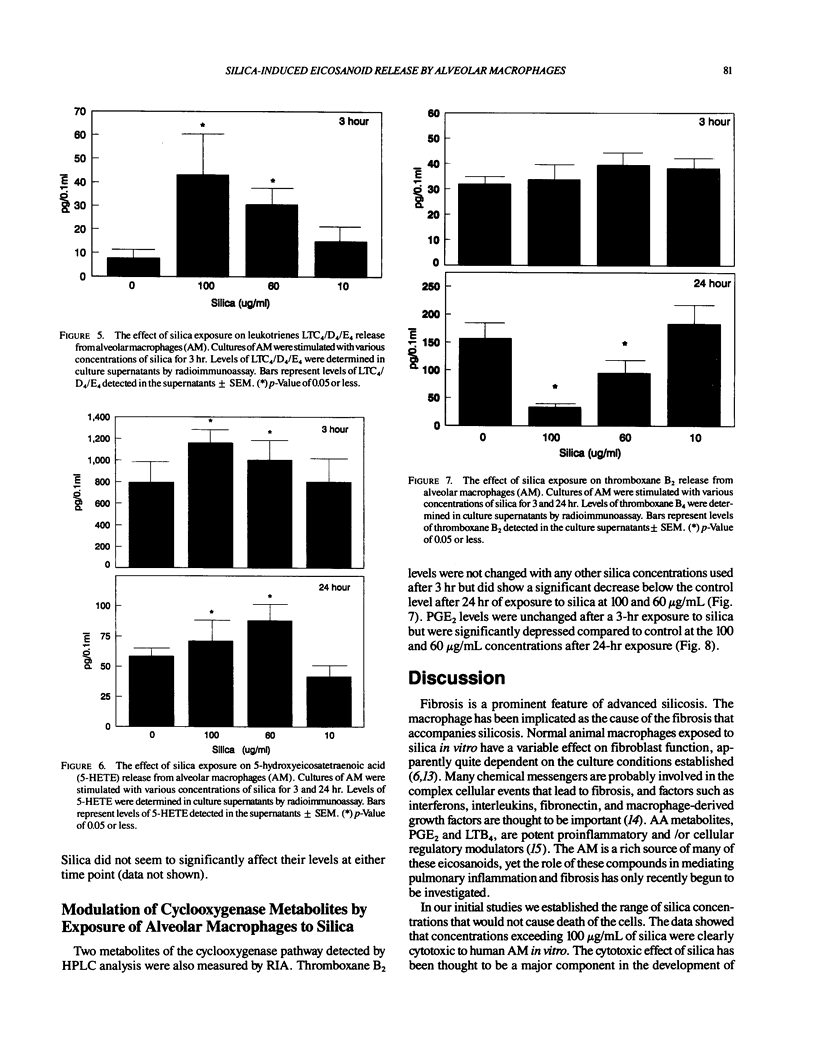
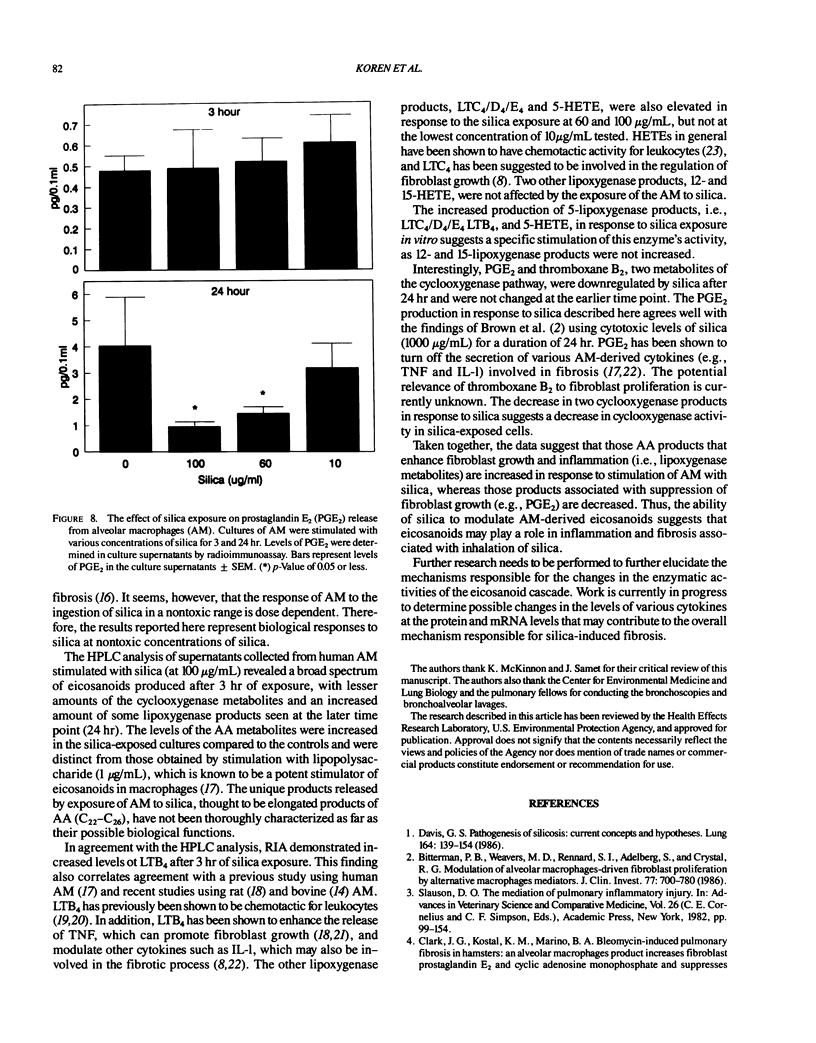
Repeated inhalation of silica dust can lead to inflammation and fibrosis in human lung and in experimental animal models. The alveolar macrophage is believed to play a pivotal role in this process. Numerous macrophage-derived growth factors, cytokines, and arachidonic acid metabolites have been shown to contribute to inflammation and fibrosis. The objective of this study was to determine the eicosanoid production by human alveolar macrophages in response to silica exposure in vitro and to assess the contribution of alveolar macrophages to silica-induced fibrosis and inflammation. Macrophages were obtained from healthy volunteers and were incubated for 3 or 24 hr in the presence of silica (100, 60, and 0 micrograms/mL). Supernatants were removed for eicosanoid analysis. Eicosanoids were analyzed by both high performance liquid chromatography and radioimmunoassay. The data suggest that silica causes an increased release of leukotriene B4, leukotrienes C4/D4/E4, and 5-hydroxyeicosatetraenoic acid (5-HETE) after 3 hr and decreases in prostaglandin E2 and thromboxane B2 production after 24 hr of exposure to 100 micrograms/mL silica. In addition, 12-HETE and 15-HETE production remained unchanged at either time point. These opposing effects seen with the metabolites of lipoxygenase and cyclooxygenase pathways could contribute to silica-induced fibrosis. The pattern of eicosanoid production after exposure to silica was different from that obtained when macrophages were stimulated with lipopolysaccharide for 3 or 24 hr, indicating that the response to the particles was not just due to general cellular activation.
Full text
PDF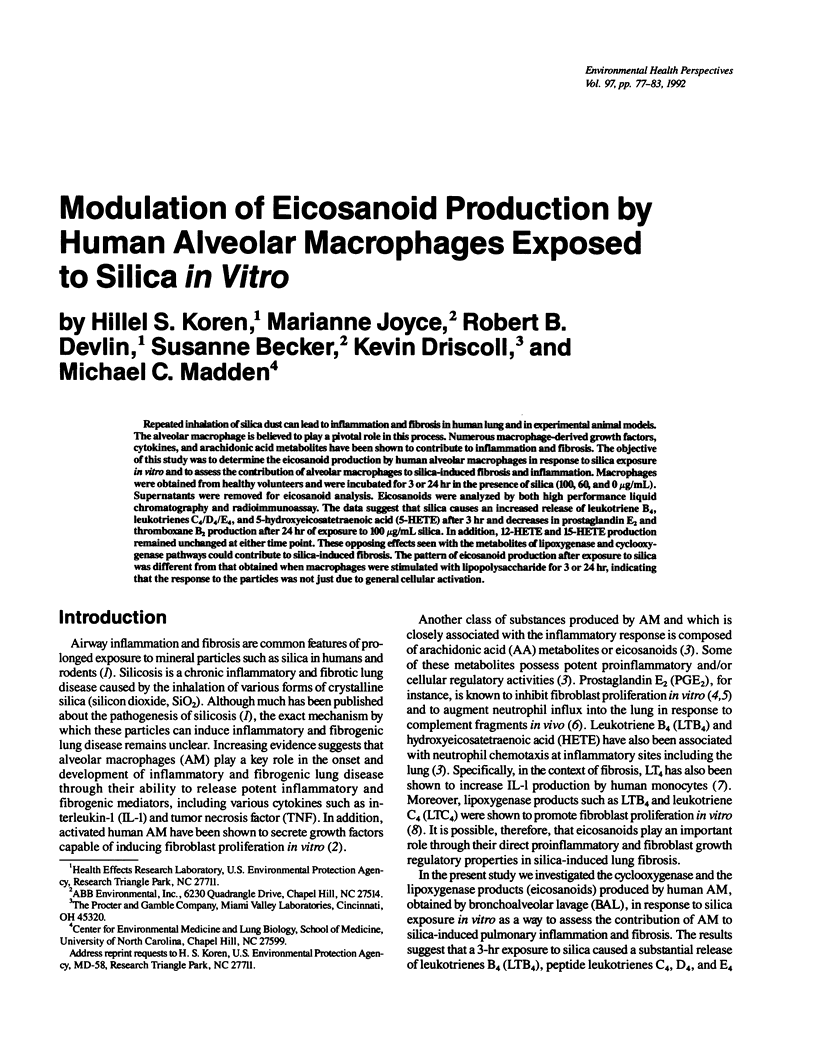

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitterman P. B., Wewers M. D., Rennard S. I., Adelberg S., Crystal R. G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986 Mar;77(3):700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H. Macrophages, dust, and pulmonary diseases. Exp Lung Res. 1987;12(2):89–107. doi: 10.3109/01902148709062834. [DOI] [PubMed] [Google Scholar]
- Brown G. P., Monick M. M., Hunninghake G. W. Human alveolar macrophage arachidonic acid metabolism. Am J Physiol. 1988 Jun;254(6 Pt 1):C809–C815. doi: 10.1152/ajpcell.1988.254.6.C809. [DOI] [PubMed] [Google Scholar]
- Brown G. P., Monick M., Hunninghake G. W. Fibroblast proliferation induced by silica-exposed human alveolar macrophages. Am Rev Respir Dis. 1988 Jul;138(1):85–89. doi: 10.1164/ajrccm/138.1.85. [DOI] [PubMed] [Google Scholar]
- Davis G. S. Pathogenesis of silicosis: current concepts and hypotheses. Lung. 1986;164(3):139–154. doi: 10.1007/BF02713638. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Gumbay R. S., Doherty D. E., LaBrecque J. F., Henson J. E., Henson P. M., Worthen G. S. Enhancement of pulmonary inflammation by PGE2: evidence for a vasodilator effect. J Appl Physiol (1985) 1988 Feb;64(2):728–741. doi: 10.1152/jappl.1988.64.2.728. [DOI] [PubMed] [Google Scholar]
- Dubois C. M., Bissonnette E., Rola-Pleszczynski M. Asbestos fibers and silica particles stimulate rat alveolar macrophages to release tumor necrosis factor. Autoregulatory role of leukotriene B4. Am Rev Respir Dis. 1989 May;139(5):1257–1264. doi: 10.1164/ajrccm/139.5.1257. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Rossman M. D., Zurier R. B., Daniele R. P. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis. 1985 Jan;131(1):94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- Englen M. D., Taylor S. M., Laegreid W. W., Liggitt H. D., Silflow R. M., Breeze R. G., Leid R. W. Stimulation of arachidonic acid metabolism in silica-exposed alveolar macrophages. Exp Lung Res. 1989 Jul;15(4):511–526. doi: 10.3109/01902148909069615. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Jr Eicosanoids and lung inflammation. Am Rev Respir Dis. 1987 May;135(5):1176–1185. doi: 10.1164/arrd.1987.135.5.1176. [DOI] [PubMed] [Google Scholar]
- Henke D., Danilowicz R., Eling T. Arachidonic acid metabolism by isolated epidermal basal and differentiated keratinocytes from the hairless mouse. Biochim Biophys Acta. 1986 Apr 15;876(2):271–279. doi: 10.1016/0005-2760(86)90284-5. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Devlin R. B., Graham D. E., Mann R., McGee M. P., Horstman D. H., Kozumbo W. J., Becker S., House D. E., McDonnell W. F. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis. 1989 Feb;139(2):407–415. doi: 10.1164/ajrccm/139.2.407. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Raugi G., Merritt T. L., Henderson W. R., Jr Relative contribution of leukotriene B4 to the neutrophil chemotactic activity produced by the resident human alveolar macrophage. J Clin Invest. 1987 Oct;80(4):1114–1124. doi: 10.1172/JCI113168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan S. H., McGarry B. M., Loeffler K. M., Kunkel S. L. Regulation of macrophage-derived fibroblast growth factor release by arachidonate metabolites. J Leukoc Biol. 1987 Aug;42(2):106–113. doi: 10.1002/jlb.42.2.106. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Lemaire I. Leukotrienes augment interleukin 1 production by human monocytes. J Immunol. 1985 Dec;135(6):3958–3961. [PubMed] [Google Scholar]
- Schmidt J. A., Oliver C. N., Lepe-Zuniga J. L., Green I., Gery I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J Clin Invest. 1984 May;73(5):1462–1472. doi: 10.1172/JCI111350. [DOI] [PMC free article] [PubMed] [Google Scholar]


