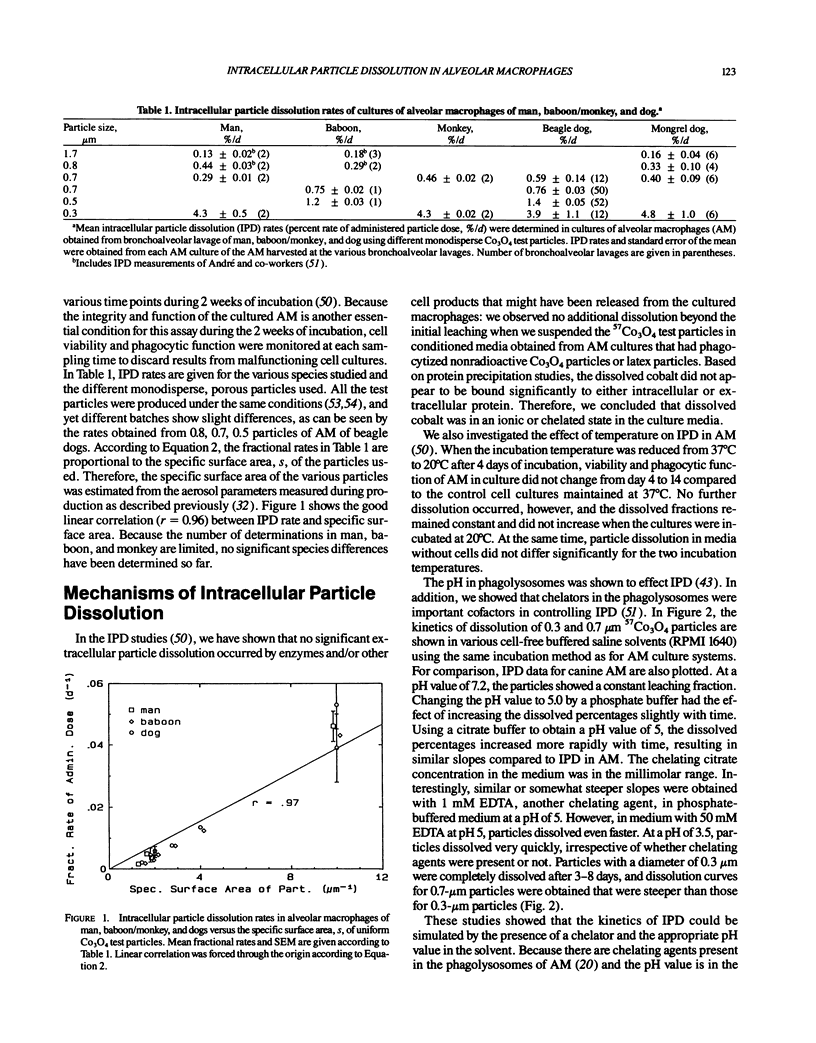
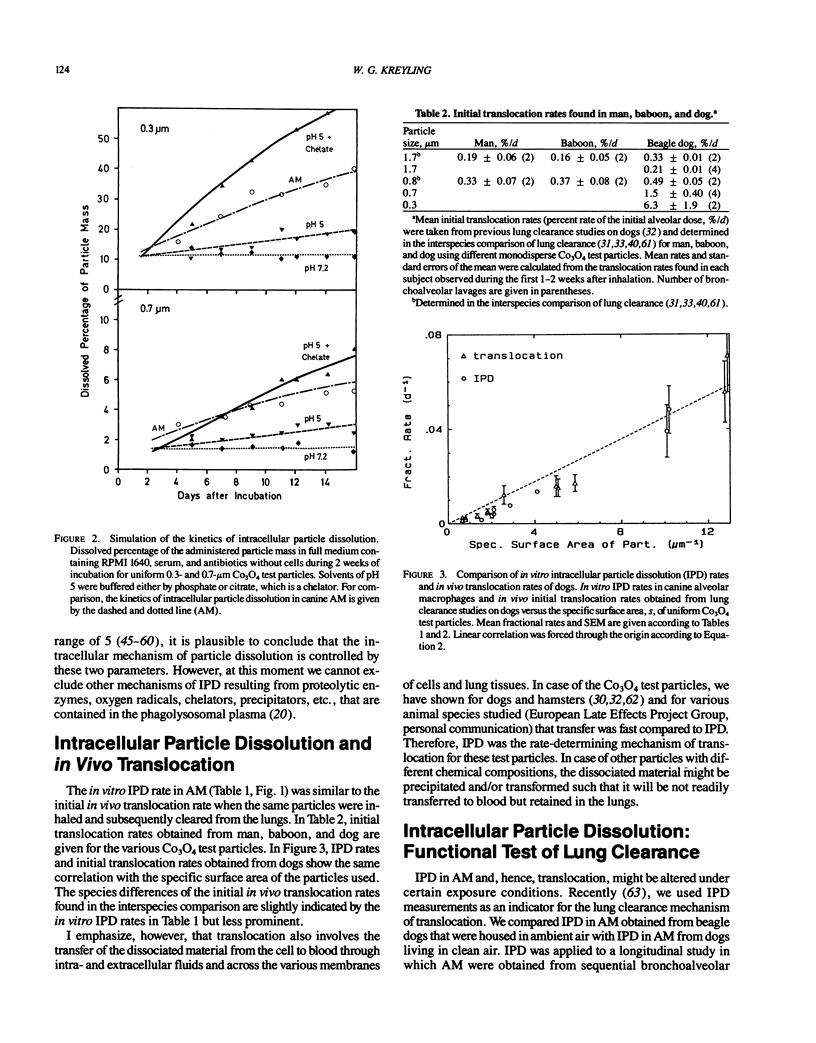
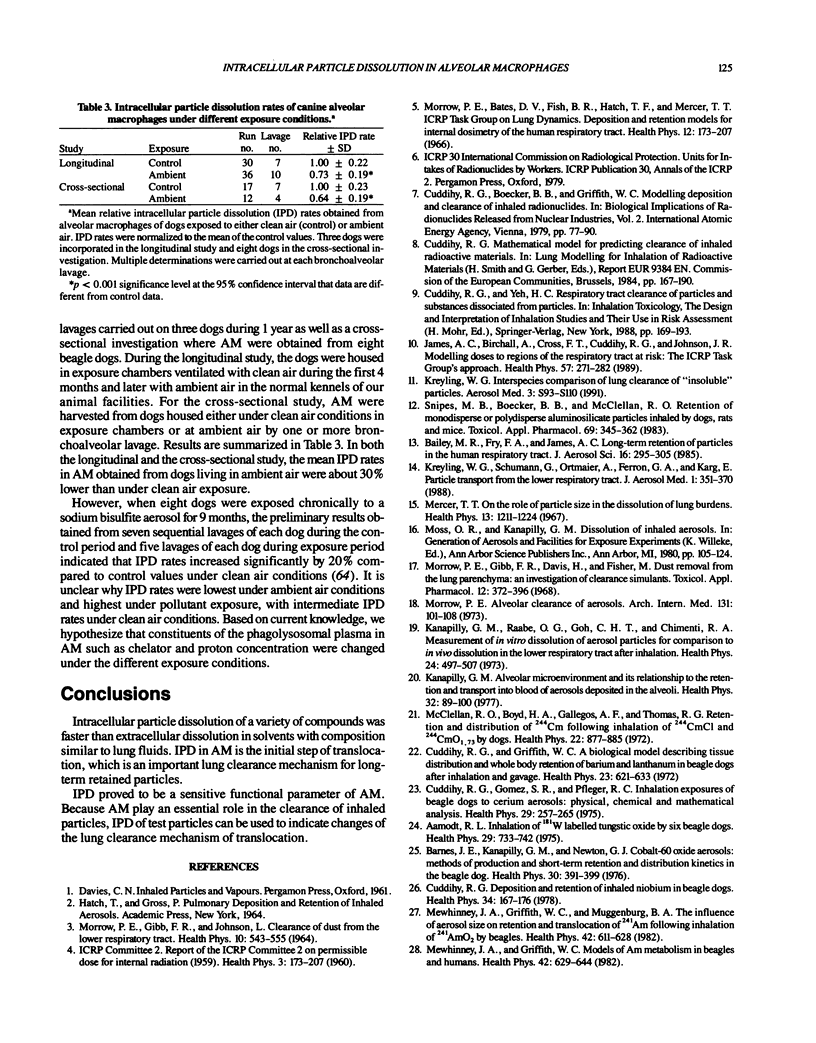
Abstract
Aerosol particles deposited in the lungs that are not readily soluble in the epithelial lining fluid will be phagocytized by alveolar macrophages (AM). Inside the phagolysosomal vacuole, the constituents of the plasma allow dissolution of a variety of compounds at a higher rate than dissolution in extracellular lung fluids. Chelator concentration and a pH value of about 5 were found to control intracellular particle dissolution (IPD). Hence, IPD is the initial step of translocation of dissolved material to blood, which is an important lung clearance mechanism for particles retained long term. IPD rates of uniform test particles determined in human, baboon, and canine AM cultures were similar to initial translocation rates determined in lung clearance studies of the same species after inhalation of the same test particles. IPD rate in cultured AM proved to be a sensitive functional parameter of AM, which was used to identify changes in the clearance mechanism of translocation during different exposure conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aamodt R. L. Inhalation of 181 W labeled tungstic oxude by six beagle dogs. Health Phys. 1975 Jun;28(6):733–743. doi: 10.1097/00004032-197506000-00010. [DOI] [PubMed] [Google Scholar]
- Barnes J. E., Kanapilly G. M., Newton G. J. Cobalt-60 oxide aerosols: methods of production and short-term retention and distribution kinetics in the beagle dog. Health Phys. 1976 May;30(5):391–398. doi: 10.1097/00004032-197605000-00003. [DOI] [PubMed] [Google Scholar]
- Bates D. V., Fish B. R., Hatch T. F., Mercer T. T., Morrow P. E. Deposition and retention models for internal dosimetry of the human respiratory tract. Task group on lung dynamics. Health Phys. 1966 Feb;12(2):173–207. [PubMed] [Google Scholar]
- Cuddihy R. G. Deposition and retention of inhaled niobium in beagle dogs. Health Phys. 1978 Feb;34(2):167–176. doi: 10.1097/00004032-197802000-00004. [DOI] [PubMed] [Google Scholar]
- Cuddihy R. G., Gomex S. R., Pfleger R. C. Inhalation exposures of beagle dogs to cerium aerosols: physical, chemical and mathematical analysis. Health Phys. 1975 Aug;29(2):257–265. doi: 10.1097/00004032-197508000-00003. [DOI] [PubMed] [Google Scholar]
- Cuddihy R. G., Griffith W. C. A biological model describing tissue distribution and whole-body retention of barium and lanthanum in beagle dogs after inhalation and gavage. Health Phys. 1972 Nov;23(5):621–633. doi: 10.1097/00004032-197211000-00004. [DOI] [PubMed] [Google Scholar]
- Ducousso R., Masse R. Vitesse de la capture précoce du lanthane par les macrophages alvéolaires. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jun 12;274(24):3328–3331. [PubMed] [Google Scholar]
- Geisow M. J., D'Arcy Hart P., Young M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981 Jun;89(3):645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J. Fluorescein conjugates as indicators of subcellular pH. A critical evaluation. Exp Cell Res. 1984 Jan;150(1):29–35. doi: 10.1016/0014-4827(84)90698-0. [DOI] [PubMed] [Google Scholar]
- James A. C., Birchall A., Cross F. T., Cuddihy R. G., Johnson J. R. The current approach of the ICRP Task Group for modeling doses to respiratory tract tissues. Health Phys. 1989;57 (Suppl 1):271–282. doi: 10.1097/00004032-198907001-00035. [DOI] [PubMed] [Google Scholar]
- Kanapilly G. M. Alveolar microenvironment and its relationship to the retention and transport into blood of aerosols deposited in the alveoli. Health Phys. 1977 Feb;32(2):89–100. doi: 10.1097/00004032-197702000-00004. [DOI] [PubMed] [Google Scholar]
- Kanapilly G. M., Raabe O. G., Goh C. H., Chimenti R. A. Measurement of in vitro dissolution of aerosol particles for comparison to in vivo dissolution in the lower respiratory tract after inhalation. Health Phys. 1973 May;24(5):497–507. doi: 10.1097/00004032-197305000-00004. [DOI] [PubMed] [Google Scholar]
- Kreyling W. G., Ferron G. A., Haider B. Metabolic fate of inhaled Co aerosols in beagle dogs. Health Phys. 1986 Dec;51(6):773–795. doi: 10.1097/00004032-198612000-00007. [DOI] [PubMed] [Google Scholar]
- Kreyling W. G., Godleski J. J., Kariya S. T., Rose R. M., Brain J. D. In vitro dissolution of uniform cobalt oxide particles by human and canine alveolar macrophages. Am J Respir Cell Mol Biol. 1990 May;2(5):413–422. doi: 10.1165/ajrcmb/2.5.413. [DOI] [PubMed] [Google Scholar]
- Lehnert B. E., Morrow P. E. Association of 59iron oxide with alveolar macrophages during alveolar clearance. Exp Lung Res. 1985;9(1-2):1–16. doi: 10.3109/01902148509061524. [DOI] [PubMed] [Google Scholar]
- Lundborg M., Eklund A., Lind B., Camner P. Dissolution of metals by human and rabbit alveolar macrophages. Br J Ind Med. 1985 Sep;42(9):642–645. doi: 10.1136/oem.42.9.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg M., Lind B., Camner P. Ability of rabbit alveolar macrophages to dissolve metals. Exp Lung Res. 1984;7(1):11–22. doi: 10.3109/01902148409087905. [DOI] [PubMed] [Google Scholar]
- MORROW P. E., GIBB F. R., JOHNSON L. CLEARANCE OF INSOLUBLE DUST FROM THE LOWER RESPIRATORY TRACT. Health Phys. 1964 Aug;10:543–555. doi: 10.1097/00004032-196408000-00003. [DOI] [PubMed] [Google Scholar]
- Marafante E., Lundborg M., Vahter M., Camner P. Dissolution of two arsenic compounds by rabbit alveolar macrophages in vitro. Fundam Appl Toxicol. 1987 Apr;8(3):382–388. doi: 10.1016/0272-0590(87)90087-x. [DOI] [PubMed] [Google Scholar]
- McClellan R. O., Boyd H. A., Gallegos A. F., Thomas R. G. Retention and distribution of 244 Cm following inhalation of 244 CmCl 3 and 244 CmO 1.73 by beagle dogs. Health Phys. 1972 Jun;22(6):877–885. doi: 10.1097/00004032-197206000-00053. [DOI] [PubMed] [Google Scholar]
- Mercer T. T. On the role of particle size in the dissolution of lung burdens. Health Phys. 1967 Nov;13(11):1211–1221. doi: 10.1097/00004032-196711000-00005. [DOI] [PubMed] [Google Scholar]
- Metivier H., Nenot J. C., Masse R., Nolibe D., Lafuma J. Etude clinique de l'épuration pulmonaire chez le singe et le chien. C R Acad Sci Hebd Seances Acad Sci D. 1974 Jan 28;278(5):671–674. [PubMed] [Google Scholar]
- Mewhinney J. A., Griffith W. C. Models of Am metabolism in beagles and humans. Health Phys. 1982 May;42(5):629–644. doi: 10.1097/00004032-198205000-00006. [DOI] [PubMed] [Google Scholar]
- Mewhinney J. A., Griffith W. C., Muggenburg B. A. The influence of aerosol size on retention and translocation of 241Am following inhalation of 241AmO2 by beagles. Health Phys. 1982 May;42(5):611–627. doi: 10.1097/00004032-198205000-00005. [DOI] [PubMed] [Google Scholar]
- Morrow P. E. Alveolar clearance of aerosols. Arch Intern Med. 1973 Jan;131(1):101–108. [PubMed] [Google Scholar]
- Morrow P. E., Gibb F. R., Davies H., Fisher M. Dust removal from the lung parenchyma: an investigation of clearance stimulants. Toxicol Appl Pharmacol. 1968 May;12(3):372–396. doi: 10.1016/0041-008x(68)90146-4. [DOI] [PubMed] [Google Scholar]
- Nilsen A., Nyberg K., Camner P. Intraphagosomal pH in alveolar macrophages after phagocytosis in vivo and in vitro of fluorescein-labeled yeast particles. Exp Lung Res. 1988;14(2):197–207. doi: 10.3109/01902148809115124. [DOI] [PubMed] [Google Scholar]
- Nyberg K., Johansson A., Camner P. Intraphagosomal pH in alveolar macrophages studied with fluorescein-labeled amorphous silica particles. Exp Lung Res. 1989;15(1):49–62. doi: 10.3109/01902148909069608. [DOI] [PubMed] [Google Scholar]
- Nyberg K., Johansson U., Rundquist I., Camner P. Estimation of pH in individual alveolar macrophage phagolysosomes. Exp Lung Res. 1989 Jul;15(4):499–510. doi: 10.3109/01902148909069614. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. L. The distribution of inhaled plutonium-239 dioxide particles within pulmonary macrophages. Arch Environ Health. 1969 Jun;18(6):904–912. doi: 10.1080/00039896.1969.10665513. [DOI] [PubMed] [Google Scholar]
- Snipes M. B., Boecker B. B., McClellan R. O. Retention of monodisperse or polydisperse aluminosilicate particles inhaled by dogs, rats, and mice. Toxicol Appl Pharmacol. 1983 Jul;69(3):345–362. doi: 10.1016/0041-008x(83)90258-2. [DOI] [PubMed] [Google Scholar]


