Abstract
Previous studies with two-dimensional gel electrophoresis techniques revealed that the cold shock response in Bacillus subtilis is characterized by rapid induction and accumulation of two classes of specific proteins, which have been termed cold-induced proteins (CIPs) and cold acclimatization proteins (CAPs), respectively. Only recently, the B. subtilis two-component system encoded by the desKR operon has been demonstrated to be essential for the cold-induced expression of the lipid-modifying desaturase Des, which is required for efficient cold adaptation of the membrane in the absence of isoleucine. At present, one of the most intriguing questions in this research field is whether DesKR plays a global role in cold signal perception and transduction in B. subtilis. In this report, we present the first genomewide transcriptional analysis of a cold-exposed bacterium and demonstrate that the B. subtilis two-component system DesKR exclusively controls the desaturase gene des and is not the cold-triggered regulatory system of global relevance. In addition to this, we identified a set of genes that might participate as novel players in the cold shock adaptation of B. subtilis. Two cold-induced genes, the elongation factor homolog ylaG and the σL-dependent transcriptional activator homolog yplP, have been examined by construction and analysis of deletion mutants.
Free-living prokaryotic organisms have the capacity to react rapidly to fluctuations of growth temperature. These responses are regulated at transcriptional and posttranscriptional levels and have been extensively characterized for heat shock, but only partially characterized for cold shock. In recent years, Bacillus subtilis has become a model organism for studies of the bacterial cold shock response representing the gram-positive branch of mesophilic soil bacteria (14).
Many reports have dealt with the function of the cold shock proteins (CSPs), a widespread protein family representing a model for the nucleic acid binding cold-shock domain (CSD). The CSD is highly conserved from bacteria to humans (15, 39, 40) and is involved in coupling transcription to translation (36). Only recently the CSDBase database was established (http://www.chemie.uni-marburg.de/∼csdbase), which includes detailed information about the CSD (37). This protein family has been identified in almost all psychrotrophic, mesophilic, thermophilic, and hyperthermophilic bacteria examined so far, and their presence in Thermotoga and Aquifex indicates an ancient origin (15). In B. subtilis, csp double-deletion strains show a variety of phenotypes, such as altered protein synthesis, aberrant nucleoid structure, cell lysis upon entry into the stationary growth phase, and impairment in sporulation (13, 39). The latter two defects were shown to be cured by heterologous expression of translation initiation factor IF1 from Escherichia coli (36).
Other investigations have revealed how B. subtilis prevents rigidification of the membrane at low temperatures. The fluidity of the membrane is maintained by isoleucine-dependent de novo synthesis of branched-chain fatty acids (20) as well as desaturation of fatty acids (1, 38), which both result in reduced attraction between adjacent fatty acid chains and hence a lower melting point.
However, so far only a little information has been available on how signal perception and transduction take place in B. subtilis after cold shock. In Synechocystis sp., the transduction of low-temperature signals was investigated by systematic disruption of histidine kinases (35). Two kinases, Hik19 and Hik33, were found to regulate the cold-induced transcription of the fatty acid desaturase genes desB and desD. In B. subtilis, a two-component system has been recently reported to regulate the desaturase gene des in a temperature-dependent manner (2). With decreasing temperature, the membrane-bound sensor kinase DesK phosphorylates its corresponding response regulator, DesR, which then binds to a specific recognition sequence in the promoter region of the des gene to activate its transcription. The activity of the membrane-located fatty acid desaturase Des finally maintains the fluidity of the membrane in the cold. This kind of signal transduction system is one example of how the bacterial cell adapts to a changing environment. Nevertheless, a general mechanism for signal transduction has not been identified so far. Therefore, it was interesting to examine whether the cold-dependent regulation by the two-component system DesK/DesR might play a global regulatory role during cold adaptation of B. subtilis rather than being restricted to regulation of the desaturase alone.
So far, most cold-induced proteins have been identified by two-dimensional gel electrophoresis (12). We used the DNA macroarray technique to examine whether or not the DesK/DesR system is of general importance for signal perception and transduction after cold shock, by determining the transcriptional profiles of genes in a B. subtilis desK deletion mutant in comparison to its parental strain, B. subtilis JH642. Moreover, this method allowed the identification of a set of significantly cold-induced genes, whose protein products might participate as novel players in the cold shock response of B. subtilis. Two of these genes were deleted, and the resulting mutants were subsequently analyzed under cold shock conditions, thereby revealing a cold-adaptive function of yplP that is similar to those of σL-dependent transcriptional activators of B. subtilis.
Construction and growth analysis of a B. subtilis desK deletion strain.
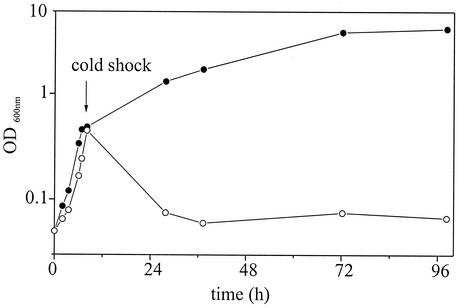
Deletion of the desaturase gene des in B. subtilis JH642 has been shown to cause a severe growth defect and to cell lysis after cold shock in the absence of isoleucine (36). Moreover, Aguilar and coworkers demonstrated that the des gene is positively controlled by the two-component system DesK/DesR in a temperature-dependent manner (2). In order to identify all genes that are transcriptionally controlled by the B. subtilis two-component system DesK/DesR, we constructed a desK deletion mutant designated B. subtilis CB10, in which a kanamycin cassette replaces an internal fragment of the desK gene (Table 1). A kanamycin cassette was amplified by PCR from plasmid pDG783 (16) with primers 5′kan783 (NcoI) and 3′kan783 (MluI) (Table 2). The purified PCR fragment was inserted into the desK gene of the MluI- and NcoI-digested plasmid pMW_Δdes (38), which contains the desK and desR genes of B. subtilis. The resulting plasmid, pCBΔdesK, was transformed into strain JH642 to give the ΔdesK kanamycin-resistant strain CB10. We analyzed the growth (optical density at 600 nm [OD600]) of CB10 at 37°C and after cold shock to 15°C (Fig. 1). In the absence of isoleucine, the desK deletion strain CB10 exhibits the same growth defect after cold shock as demonstrated for the des deletion strain (38).
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| B. subtilis | ||
| JH642 | pheA1 sfp0trpC2 | Hoch and Mathews (16a) |
| CB10 | JH642 desK::kan | This work |
| CB15 | JH642 yplP::kan | This work |
| CB16 | JH642 ylaG::kan | This work |
| E. coli XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10(Tetr)] | Stratagene |
TABLE 2.
PCR primers used in this study
| Primer | Sequencea |
|---|---|
| 5′kan783 (NcoI) | TATCCATGGAGGTGATAGGTAAGATTATA |
| 3′kan783 (MluI) | TTAACGCGTCTAGAGTCGATACAAATTC |
| 5′kan783 (ClaI) | ATAATCGATAGGTGATAGGTAAGATTATAC |
| 3′kan783 (ClaI) | ATAATCGATTAAAACATCAGAGTATGGACA |
| yplP −455 (EcoRI) | ATAGAATTCTTTCATTTTATGATAAGCCCC |
| yplP + 1475 (EcoRI) | TATGAATTCTCAGTGAAGCATATAAAGGTT |
| ylaG_P1 | AACAGCGGAGAAAAATTGTTCAAGTGCAA |
| ylaG_P2 | CGAGCTCGAATTCGTAATCATGGTCATATCATATTATATCACACATCCTCTTTAAAAGC |
| ylaG_P3 | GTATAATCTTACCTATCACCTCAAATGGTTGAATTTGAAAAGATAGAACCCGTACGTTTA |
| ylaG_P4 | ATCCGGTAAGAGGTATACTGGATGAATTA |
| 5′des_Sonde | TGATTCAGCTTTTAAACACGT |
| 3′des_Sonde + T7 | TAATACGACTCACTATAGGGAGAATCTTCAAAGGTATGCT |
| 5′ylaG_Sonde | CAATGGACTCTAATGATCTTG |
| 3′ylaG_Sonde + T7 | TAATACGACTCACTATAGGGCGTCAAGCTTCATAAGAGAAA |
| 5′yplP_Sonde | AGCTTCCTGTCTTAATAACAG |
| 3′yplP_Sonde + T7 | TAATACGACTCACTATAGGGTTGCTGTGTTCAGAAATGATC |
Cutting sites are in boldface. T7 polymerase promoters are underlined.
FIG. 1.
Growth of desK deletion strain B. subtilis CB10 in the presence (•) and absence (○) of isoleucine (50 μg/ml). Cells were grown in SMMTrpPhe minimal medium (36) supplemented with 0.5% (wt/vol) glucose, trace elements, 50 μg of tryptophan per ml, and 50 μg of phenylalanine per ml at 37°C to an OD600 of 0.45 and then subjected to cold shock (15°C).
Northern blot analysis of the des gene of B. subtilis.
Prior to macroarray analysis, we performed Northern blot experiments (32) with total mRNA isolated as described before (27). For strain JH642 grown in SMMTrpPheIle (36), a maximum of des transcription was detected 70 min after cold shock by Northern blot analysis (data not shown). Assuming significant transcriptional induction of DesK/DesR-regulated genes in general under these conditions, all further experiments were carried out with samples taken 70 min after cold shock. As expected, the Northern blot showed no transcription of des in the desK deletion strain CB10 under any conditions (data not shown). As a control, the transcription of the groEL gene was monitored. The groEL gene encodes the class I heat shock protein GroEL, which is involved in the folding of proteins after heat shock (29). The transcription of groEL in JH642 was strongly repressed 70 min after cold shock (Fig. 2), which is in agreement with previous results (19). These total mRNA samples were used for all further experiments in this study.
FIG. 2.
Northern blot analysis of des and groEL genes of B. subtilis JH642. Experiments were performed with primer pairs 5′des_Sonde and 3′des_Sonde+T7, and 5′groEL_Sonde and 3′groEL_Sonde+T7 to generate a des-specific probe and a groEL-specific probe. Cells were grown in SMMTrpPheIle minimal medium at 37°C to an OD600 of 0.45 and then subjected to cold shock (15°C). Samples were taken at 37°C immediately prior to cold shock and 70 min after shock at 15°C. Total mRNA was isolated from cells and analyzed to determine the amounts of des and groEL transcript. Lanes: M, marker; 1, des, 37°C; 2, des, 15°C; 3, groEL, 37°C; 4, groEL, 15°C. Transcription of des is induced at low temperatures, and transcription of groEL is repressed at low temperatures.
Transcriptional profiling of B. subtilis JH642 and its desK deletion derivative, CB10.
It was demonstrated that transcription of the B. subtilis des gene is specifically cold induced by DesK/DesR (2). This finding represented a molecular thermosensor in B. subtilis and gave rise to the question of whether des is the only gene or whether this two-component system might participate as the key regulatory system for the B. subtilis cold shock response. Since regulation by DesK/DesR takes place at the transcriptional level, the use of DNA macroarrays appeared to be the appropriate method for investigation. Experiments were performed as described by Petersohn et al. (30). For cDNA synthesis, 2 μg of total RNA was mixed with 4 μl of a commercially available primer mix, which consisted of 4,107 specific oligonucleotide primers complementary to the 3′ ends of all B. subtilis mRNAs (Sigma-Genosys, Ltd.). This study was performed with Panorama B. subtilis gene arrays from Sigma-Genosys, Ltd., which carry duplicate spots of PCR products representing the 4,107 known B. subtilis genes. Hybridization signals were detected by PhosphorImager and quantified with ArrayVision software (version 6.0; Imaging Research, Inc.). Further analysis was carried out with GeneSpring (version 4.2; Silicon Genetics). Genes that are exclusively controlled by DesK/DesR are not induced in desK deletion strain CB10. Filter hybridizations of three independent sample preparations clearly showed that desK, desR, and des were the only genes that are not transcribed in CB10 after cold shock from 37°C to 15°C compared to the parental strain, JH642. The lack of transcriptional signal for desK and desR in CB10 after cold shock is due to the nature of the mutation introduced into the desKR operon. The absence of a signal for the des gene in CB10 after cold shock shows that it is the only gene exclusively controlled by the DesKR system after cold shock. Hence, DesK/DesR does not represent the general temperature perception system for induction of the cold shock response in B. subtilis. In a recent study by Kobayashi et al., DesK and DesR were examined along with 23 other two-component systems in B. subtilis (21). Under the conditions tested, 28 genes were regulated by the DesK/DesR system, including des and the desKR operon. This discrepancy might be explained by the different experimental conditions used. We applied the physiological stimuli for the DesK/DesR system (temperature shift and membrane fluidity), whereas Kobayashi et al. overproduced the response regulator DesR in the absence of the sensor kinase DesK at 37°C (21).
Identification of novel players during cold adaptation in B. subtilis.
Comparison of mRNA levels of strain JH642 grown in SMMTrpPheIle before and 70 min after cold shock by DNA macroarrays established a global overview about all cold-regulated genes in B. subtilis. Among the 80 genes induced (more than twofold) by cold shock, we identified 40 genes of unknown function, which might represent novel players during the cold shock response in B. subtilis. We tried to find new conserved upstream regulatory regions by bioinformatic studies, but the results were not significant (data not shown). A larger number of genes, 280, were repressed upon cold shock (more than twofold). Table 3 shows a selection of the most significant results obtained in this study. The complete data have been stored in a searchable form on the internet and can be accessed through CSDBase at http://www.chemie.uni-marburg.de/∼csdbase (37).
TABLE 3.
Transcriptional profiling of B. subtilis JH642 after cold shock from 37°C to 15°Ca
| Gene (15°C/37°C ratio)b | Gene product (function) |
|---|---|
| ydjO (6.2) | Unknown |
| ylaG (3.3) | Putative GTP binding elongation factor |
| yplP (8.1) | Putative σL-dependent transcriptional regulator |
| des (10.7) | Fatty acid desaturase |
| desK (11.9) | Two-component sensor kinase (activation of des) |
| desR (18.5) | Two-component regulator (activation of des) |
| cspB (2.3) | Major CSP |
| cspC (9.1) | CSP |
| cspD (1.9) | CSP |
| ydbR (2.6), yqfR (2.3) | Putative DEAD box helicases |
| rbfA (2.6) | Ribosomal binding factor |
| rplE (2.3), rplF (2.7), rplN (2.1), rplR (3.2), rplX (2.3), rpmD (2.2), rpmJ (2.0), | Ribosomal proteins |
| rpsE (2.5), rpsH (3.0), rpsM (2.7), rpsN (2.1) | |
| infA (2.3), infB (2.1) | Initiation factors IF1 and IF2 |
| ytrA (4.8), ytrB (2.2), ytrC (5.2), ytrD (4.5), ytrE (5.8), ytrF (7.5) | ABC transporter (acetoin utilization) |
| gyrA (2.1), gyrB (2.2) | DNA gyrase (negative supercoil) |
| topA (0.5) | DNA topoisomerase I (relaxes negative supercoil) |
| hrcA (0.3) | Transcriptional regulator (CIRCE regulator) |
| grpE (0.2), dnaK (0.2), dnaJ (0.3), yqeT (0.4), yqeU (0.5) | Chaperones (class I heat shock genes) |
| groEL (0.2), groES (0.1) | Chaperones (class I heat shock genes) |
| clpP (0.4) | Clp protease subunit (class III heat shock gene) |
| ahpC (0.4), ahpF (0.4) | Alkyl hyperoxide reductase (class IV heat shock genes) |
| argB (0.3), argC (0.8), argD (0.4), argG (0.5), argH (0.4), argJ (0.2), aroA (0.1), | Amino acid biosynthesis |
| aroB (0.5), aroF (0.3), aroH (0.4), asd (0.3), aspB (0.3), carA (0.4), dapB (0.5), dapG (0.4), glnA (0.3), gltA (0.3), gltB (0.3), glyA (0.4), hom (0.5), ilvD (0.5), metE (0.3), proB (0.4), proH (0.4), serA (0.1), serC (0.2), thrC (0.5) | |
| aspS (0.4), hisS (0.2), metS (0.4), thrS (0.5) | tRNA synthetases |
| purF (0.5), purN (0.5), purQ (0.3), purL (0.3), purM (0.6), purK (0.2), purE | Purine biosynthesis |
| (0.2), purC (0.3), purB (0.2), guaB (0.2), ndK (0.4) | |
| pyrA (0.5), pyrB (0.7), pyrC (0.4) | Pyrimidine biosynthesis |
| nifS (0.4), nadA (0.3), nadB (0.5), nadC (0.4) | NAD biosynthesis |
| upp (0.4) | Uracil phosphoribosyltransferase (pyrimidine salvage) |
| prs (0.3) | Phosphoribosyl pyrophosphate synthetase |
| pgi (0.4), pgk (0.5), tpi (0.4) | Glycolysis |
| pdhA (0.2), pdhB (0.3), pdhC (0.5), pdhD (0.5) | Pyruvate dehydrogenase |
| sucC (0.4), sdhC (0.4), citG (0.5) | Citric acid cycle |
| atpA (0.3), alpB (0.1), atpE (0.2), atpF (0.2), atpH (0.3), atpI (0.4) | ATP synthase |
The genes listed in the table are described in the text. A complete list containing the transcriptional patterns of all genes is available on the internet at http://www.chemie.uni-marburg.de/∼csdbase (42).
Hybridization signals from three filter hybridizations of independently grown cultures were detected by PhosphorImager and quantified with ArrayVision software (version 6.0; Imaging Research, Inc.). Further analysis was carried out with GeneSpring (version 4.2; Silicon Genetics). The average correlation factor of the three respective parallel experiments was 0.99146.
CSPs.
CSPs represent the highly induced proteins after an abrupt downshift in growth temperature. In B. subtilis, there exist three homologs of the widespread and highly conserved CSP family, CspB, CspC, and CspD, of which at least one copy is essential for survival even under optimal growth conditions (13). It was shown previously that CSPs bind to single-stranded DNA and RNA (11, 31) and colocalize with ribosomes in vivo (28, 39). Recently, the growth phenotype as well as a sporulation defect in a cspB cspC double-deletion strain could be cured by expression of translation initiation factor IF1 from E. coli, indicating an overlapping function of both protein classes (36). CSP induction after cold shock mainly occurs at the posttranscriptional level, whereas transcriptional activation of csp genes is only modest (19). Our macroarray analyses showed the strongest induction for the cspC gene (9.1-fold), a moderate induction of cspB (2.3-fold), and low induction for cspD (1.9-fold) (Table 3).
Heat shock genes.
Our results demonstrate repression of a wide range of heat shock genes, indicating that cold and heat shock genes are often regulated antagonistically. Both operons of class I heat shock genes (hrcA, grpE, dnaK, dnaJ, yqeT, and yqeU, as well as groEL and groES) were repressed three- to sevenfold (Table 3). Class I heat shock genes are usually induced by the HrcA-CIRCE regulation mechanism after occurrence of heat-denaturated proteins (29). Heat shock genes of classes III (clpP) and IV (ahpC and ahpF) were also repressed (threefold) at the transcriptional level. Proteomic approaches show that heat shock proteins GroES and ClpP are repressed upon cold shock, as reported in studies using two-dimensional gel electrophoresis (12). It seems reasonable that B. subtilis has no need for heat shock proteins at low temperatures and can save valuable resources by repressing the corresponding genes.
Translation machinery.
A set of genes encoding ribosomal proteins (names given in parentheses) were two- to threefold cold induced (Table 3), such as rplE (L5), rplF (L6), rplN (L14), rplR (L18), rplX (L24), rpmD (L30), rpmJ (L36), rpsE (S5), rpsH (S8), rpsM (S13), and rpsN (S14). Our studies show that not all ribosomal components are synthesized de novo upon cold shock. Rather, it appears that a selected subset of ribosomal components is required, whose individual functions might have special importance after cold shock. Furthermore, infA and infB, encoding initiation factor homologs, were twofold induced. Another effect on translation initiation might be the twofold induction of the rbfA gene of B. subtilis upon cold shock, since RbfA of E. coli was shown to bind to the 30S subunit of the ribosome and might effect translation initiation at low temperatures (7). Eventually translation elongation might be adapted at low temperatures as well, since the yet uncharacterized ylaG gene, which possibly encodes a GTP binding elongation factor homolog, was threefold induced (Table 3).
Efficient translation requires adequate mRNA templates, whose ribosomal binding sites are not masked due to formation of secondary structures, which are more stable at low temperatures. CSPs are thought to couple transcription and translation by low-affinity occupation of nascent transcripts in order to prevent the formation of mRNA secondary structure (13). Furthermore, RNA helicases might play an active role in reverting such structures, as has been shown for E. coli CsdA (18). Cold-induced RNA helicases were reported for the gram-negative bacterium E. coli (csdA) (18), the cyanobacterium Anabaena sp. (chrC) (6), and the Antarctic archaeon Methanococcoides burtonii (deaD) (25). They are involved in unwinding double-stranded RNA due to helix-destabilizing activity (18) and thereby possibly facilitate initiation of translation. Indeed, we identified two cold-induced genes, ydbR (2.6-fold) and yqfR (2.3-fold), that appear to encode B. subtilis RNA helicases homologous to E. coli CsdA. The products encoded by ydbR and yqfR might represent novel players during cold adaptation of B. subtilis. It is worth noting that ribosomal binding factor RbfA (pI 9.3) and the two helicases mentioned above (both pI 9.9) were not identified during the two-dimensional gel electrophoresis studies reported earlier (12) because of the pH range employed (pH 4 to 7).
Nucleoid structure.
Topology of DNA is generally important for transcription. The DNA gyrase introduces a negative supercoil, which has been demonstrated to have a regulatory function for gene expression in the cold, since addition of gyrase inhibitor novobiocin abolishes cold induction of the fatty acid desaturase gene des (10). In the case of B. subtilis, more detailed investigations addressing the role of topoisomerase proteins during cold adaptation have not been carried out so far. However, our present results show that the gyrA and gyrB genes encoding DNA gyrase were twofold induced, whereas the topA gene encoding DNA topoisomerase I was twofold repressed. This antagonistic regulation likely results in the previously observed negative supercoil of DNA after cold shock (22).
ABC transporter.
The Ytr ABC transporter has been proposed to play a role in acetoine utilization (42). B. subtilis produces acetoin as an external carbon storage compound during glucose excess and reutilizes it later during the stationary phase and sporulation (26). The complete operon (ytrA, ytrB, ytrC, ytrD, ytrE, and ytrF) was four- to sevenfold induced after cold shock (Table 3). The first gene encodes a repressor, and the five genes that follow encode the ABC transporter. Acetoine (3-hydroxy 2-butanone) is of potential interest for cold adaptation, because it is easily converted to 2,3-butanediol, and polyols, along with other polar molecules such as polyamines and sugars, have been described as cryoprotectants (23, 41). Thus, acetoin or derivatives might have a possible role as cryoprotectants for B. subtilis.
Amino acid biosynthesis.
One of the obvious changes after cold shock was the negative regulation of many amino acid biosynthetic pathways. The transcriptional level of 31 genes required for the biosynthesis of nonpolar (Gly, Val, Met, and Ile), polar (Ser, Thr, Pro, and Gln), charged (Arg, His, and Asp), and aromatic (Phe, Tyr, and Trp) amino acids was reduced (Table 3). In addition, four genes encoding tRNA synthetases (aspS, hisS, metS, and thrS) were two- to fourfold repressed as well. The slower growth results in a generally reduced protein synthesis after cold shock (12), which might result in an oversupply of amino acids that leads to a feedback inhibition of genes associated with amino acid biosynthesis.
Purine and/or pyrimidine biosynthesis.
Many operons involved in the purine/pyrimidine biosynthetic pathways were downregulated after cold shock. Genes encoding proteins involved in the biosynthesis of inosine 5′monophosphate and, thereafter, GTP and ATP were repressed (purF, purN, purQ, purL, purM, purK, purE, purC, purB, guaB, and ndk). The same pattern was obtained for the biosynthesis of the pyrimidines UTP and CTP (pyrA, pyrB, pyrC, smbA, and ndk). Slower growth after cold shock would also mean slower DNA replication and therefore a reduced need for nucleotides. Not only were the genes for nucleotides repressed but also those for the biosynthesis of the cofactor NAD (nifS, nadB, nadA, and nadC), a gene for pyrimidine salvage (upp), and the prs gene of B. subtilis, encoding the PRPP synthetase, which is the starting point for purine/pyrimidine synthesis (4).
Glycolysis, citric acid cycle, and ATP synthesis.
After cold shock, some genes involved in glycolysis (pgi, pgk, and tpi), pyruvate dehydrogenase (pdhA, pdhB, pdhC, and pdhD), and the citric acid cycle (sucC, sdhC, and citG) were repressed. In addition, transcription of the ATP synthase operon (atpA, atpB, atpE, atpF, atpH, and atpI) was downregulated. Like the reduced amino acid and purine/pyrimidine biosynthesis, the repression of these genes encoding for enzymes with central metabolic functions points at the overall reduced metabolic activity of B. subtilis after cold shock.
Construction and analysis of deletion strains of cold-induced genes ylaG and yplP.
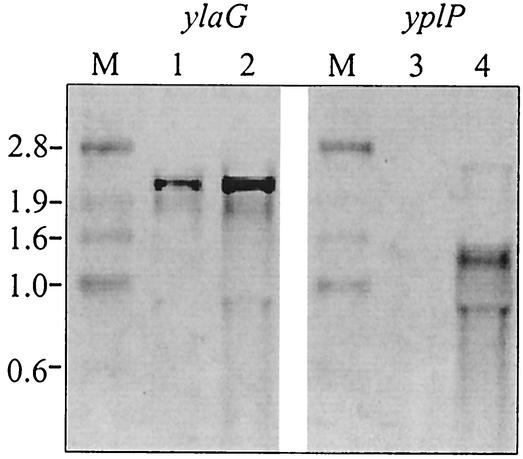
To investigate a possible function of uncharacterized cold-induced genes (Table 3), we constructed and analyzed deletion mutants of ylaG (3.3-fold induced) and yplP (8.1-fold induced). First the data obtained from the macroarray analysis were confirmed by Northern blot analysis. A significant cold induction was detected for both ylaG and yplP mRNA (Fig. 3). The B. subtilis ylaG deletion strain CB16 was constructed basically by the method described by Kuwayama et al. (24). Two sets of primers, ylaG_P1 and ylaG_P2 and ylaG_P3 and ylaG_P4, were used to amplify the 5′ and 3′ flanking regions of the ylaG gene, respectively. Primers ylaG_P2 and ylaG_P3 contained sequences that are identical to the ends of the kanamycin cassette of plasmid pDG783. In a second PCR, the previously amplified 5′ and 3′ flanking regions, which contain the terminal kanamycin regions, were used as primers to incorporate the kanamycin cassette between the two ylaG flanking regions. The resulting product was boosted by PCR with the primers ylaG_P1 and ylaG_P4 to give a ready-to-use deletion fragment of 3.6 kbp carrying the resistance cassette between the flanking regions, which was transformed into B. subtilis strain JH642 without further purification.
FIG. 3.
Northern blot analysis of ylaG and yplP genes of B. subtilis JH642. Cells were grown in SMMTrpPheIle minimal medium at 37°C to an OD600 of 0.45 and then subjected to cold shock (15°C). Experiments were performed with primer pairs 5′ylaG_Sonde and 3′ylaG_Sonde+T7 and 5′yplP_Sonde and 3′yplP_Sonde+T7 to generate a ylaG-specific probe and a yplP-specific probe. Samples were taken immediately prior to cold shock at 37°C and 70 min after shock at 15°C. Total mRNA was isolated from cells and analyzed for the amounts of ylaG and yplP transcript. Lanes: M, marker; 1, ylaG, 37°C; 2, ylaG, 15°C; 3, yplP, 37°C; 4, yplP, 15°C. Both genes show an increased amount of transcript at low temperatures.
For the construction of yplP deletion strain CB15, the yplP gene including flanking regions was amplified by PCR from chromosomal DNA of B. subtilis JH642 with primers yplP−455(EcoRI) and yplP+1475(EcoRI). The PCR fragment was cloned into the EcoRI site of plasmid pQE70 (34), resulting in plasmid pQEyplP. A kanamycin cassette was amplified by PCR from plasmid pDG783 with primers 5′kan783 (ClaI) and 3′kan783 (ClaI), and the fragment obtained was inserted into the ClaI site of pQEyplP to give the deletion plasmid pCBΔyplP. The linearized pCBΔyplP was used to transform B. subtilis JH642 to give CB15.
ylaG was chosen, because a BLASTP search (3) revealed three conserved domains that share significant similarity with GTP-binding elongation factors. The N terminus of this protein family typically contains a GTP binding domain (P-loop motif), whereas the C terminus contains two β-barrel structures, the first of which binds to amino-acid-charged tRNA. The product encoded by the ylaG gene shares 37% identity (52% similarity) with EF-G and 30% identity (45% similarity) with EF-Tu from B. subtilis. Since many of the previously reported cold-induced proteins (12), as well as a good fraction of the cold-induced genes identified in this study, are involved in ribosomal function, the similarity to elongation factors EF-G and EF-Tu encouraged us to further analyze whether the ylaG gene product might have a function during the elongation process at low temperatures. However, a growth analysis of CB16 under cold shock conditions revealed no significant difference in growth rate compared to that of the parental strain JH642 at 15°C. This shows that ylaG removal has no impact on growth after cold shock and is not essential for B. subtilis. To further examine a possible function of ylaG in cold adaptation, detailed studies of CB16 by two-dimensional gel electrophoresis have been initiated.
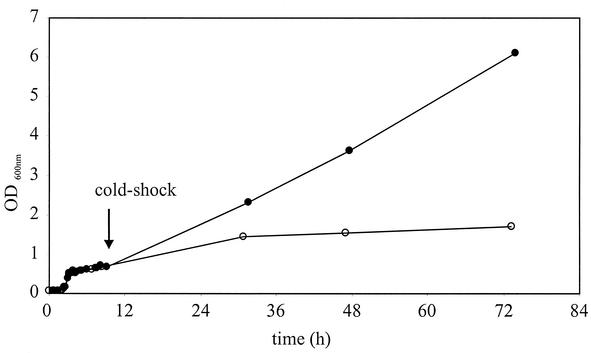
In the case of the second gene under investigation, yplP, a BLASTP search (3) revealed significant similarity to the E. coli NtrC/NifA family of transcriptional regulators. In B. subtilis, five homologs to this protein family are known, of which the following four have been described. AcoR (31% identity, 48% similarity) (17), BkdR (32% identity, 50% similarity) (8), LevR (25% identity, 43% similarity) (9), and RocR (31% identity, 47% similarity) (5) interact with σL (σ54) as transcriptional enhancers of operons involved in carbohydrate metabolism and amino acid catabolism. The sequence alignment of YplP shows the well-conserved σ54 interaction domain as well as the C-terminal DNA binding motif (data not shown). Interestingly, the typical N-terminal domain that is responsible for signal transduction in other σL/σ54 activators (33) is not present in YplP, indicating that this protein might be triggered by a different stimulus compared to its homologs. To test the in vivo significance of the yplP gene product as a possible transcriptional regulator during cold shock adaptation, the yplP deletion strain CB15 was grown under cold shock conditions. The results presented in Fig. 4 revealed a late-growth phenotype for the mutant compared to the parental strain after prolonged incubation at low temperatures. This cold-specific late-growth phenotype suggests that the yplP gene product may have a role during cold adaptation of B. subtilis. To characterize the role as a thermosensing transcriptional activator, possibly by interaction of YplP with σL, further investigations, including studies to identify potential genes controlled by YplP and their role in cold shock adaption, are under way.
FIG. 4.
Growth of yplP deletion strain B. subtilis CB15 (○) and parental strain B. subtilis JH642 (•) in Luria-Bertani medium. Cells were grown at 37°C to an OD600 of 0.45 and then subjected to cold shock (15°C).
It is surprising that the σL-dependent transcriptional activator bkdR is not induced upon cold shock, like what we showed for yplP, since BkdR activates a metabolic pathway for isoleucine degradation (8). This pathway forms precursors for the isoleucine-dependent de novo synthesis of anteiso branched-chain fatty acids, which have been shown to be cold protective (20). Anteiso branched-chain fatty acids lower the melting point of the membrane like unsaturated fatty acids produced by the desaturase des (38) to maintain membrane fluidity. Further investigations are necessary to examine whether the lack of bkdR induction upon cold shock is associated with the known but yet uncharacterized defect in isoleucine metabolism of strain B. subtilis JH642 (20).
In summary, low temperatures result in a stress response of B. subtilis that is characterized by strong repression of major cellular metabolic activities, whereas only a limited number of processes essential for cold adaptation are induced. These include proteins associated with the translation machinery and membrane adaptation, for which activation through thermosensing systems is necessary. Although the role of the thermosensing two-component system DesKR has been characterized, and the function of the potential σL-dependent transcriptional activator YplP has yet to be defined, a global cold sensor in B. subtilis is still missing.
Acknowledgments
We thank Nadine Kessler and Peter Graumann for critical reading of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) and Fonds der Chemischen Industrie.
REFERENCES
- 1.Aguilar, P. S., J. E. Cronan, Jr., and D. de Mendoza. 1998. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J. Bacteriol. 180:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar, P. S., A. M. Hernandez-Arriaga, L. E. Cybulski, A. C. Erazo, and D. de Mendoza. 2001. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 20:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Arnvig, K., B. Hove-Jensen, and R. L. Switzer. 1990. Purification and properties of phosphoribosyl-diphosphate synthetase from Bacillus subtilis. Eur. J. Biochem. 192:195-200. [DOI] [PubMed] [Google Scholar]
- 5.Calogero, S., R. Gardan, P. Glaser, J. Schweizer, G. Rapoport, and M. Debarbouille. 1994. RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J. Bacteriol. 176:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamot, D., W. C. Magee, E. Yu, and G. W. Owttrim. 1999. A cold shock-induced cyanobacterial RNA helicase. J. Bacteriol. 181:1728-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dammel, C. S., and H. F. Noller. 1995. Suppression of a cold-sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes Dev. 9:626-637. [DOI] [PubMed] [Google Scholar]
- 8.Debarbouille, M., R. Gardan, M. Arnaud, and G. Rapoport. 1999. Role of bkdR, a transcriptional activator of the sigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 181:2059-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debarbouille, M., I. Martin-Verstraete, A. Klier, and G. Rapoport. 1991. The transcriptional regulator LevR of Bacillus subtilis has domains homologous to both sigma 54- and phosphotransferase system-dependent regulators. Proc. Natl. Acad. Sci. USA 88:2212-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grau, R., D. Gardiol, G. C. Glikin, and D. de Mendoza. 1994. DNA supercoiling and thermal regulation of unsaturated fatty acid synthesis in Bacillus subtilis. Mol. Microbiol. 11:933-941. [DOI] [PubMed] [Google Scholar]
- 11.Graumann, P., and M. A. Marahiel. 1994. The major cold shock protein of Bacillus subtilis CspB binds with high affinity to the ATTGG- and CCAAT sequences in single stranded oligonucleotides. FEBS Lett. 338:157-160. [DOI] [PubMed] [Google Scholar]
- 12.Graumann, P., K. Schröder, R. Schmid, and M. A. Marahiel. 1996. Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 178:4611-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graumann, P., T. M. Wendrich, M. H. Weber, K. Schröder, and M. A. Marahiel. 1997. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol. Microbiol. 25:741-756. [DOI] [PubMed] [Google Scholar]
- 14.Graumann, P. L., and M. A. Marahiel. 1999. Cold shock response in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1:203-209. [PubMed] [Google Scholar]
- 15.Graumann, P. L., and M. A. Marahiel. 1998. A superfamily of proteins that contain the cold-shock domain. Trends Biochem. Sci. 23:286-290. [DOI] [PubMed] [Google Scholar]
- 16.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 16a.Hoch, J. A., and J. Mathews. 1973. Chromosomal location of pleiotropic sporulation mutations in Bacillus subtilis. Genetics 73:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, M., F. B. Oppermann-Sanio, and A. Steinbüchel. 1999. Biochemical and molecular characterization of the Bacillus subtilis acetoin catabolic pathway. J. Bacteriol. 181:3837-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, P. G., M. Mitta, Y. Kim, W. Jiang, and M. Inouye. 1996. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc. Natl. Acad. Sci. USA 93:76-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaan, T., B. Jurgen, and T. Schweder. 1999. Regulation of the expression of the cold shock proteins CspB and CspC in Bacillus subtilis. Mol. Gen. Genet. 262:351-354. [DOI] [PubMed] [Google Scholar]
- 20.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, K., M. Ogura, H. Yamaguchi, K.-I. Yoshida, N. Ogasawara, T. Tanaka, and Y. Fujita. 2001. Comprehensive DNA microarray analysis of Bacillus subtilis two-component regulatory systems. J. Bacteriol. 183:7365-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krispin, O., and R. Allmansberger. 1995. Changes in DNA supertwist as a response of Bacillus subtilis towards different kinds of stress. FEMS Microbiol. Lett. 134:129-135. [DOI] [PubMed] [Google Scholar]
- 23.Kushad, M. M., and G. Yelenosky. 1987. Evaluation of polyamine and proline levels during low temperature acclimation of citrus. Plant Physiol. 84:692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwayama, H., S. Obara, T. Morio, M. Katoh, H. Urushihara, and Y. Tanaka. 2002. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res. 30:E2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim, J., T. Thomas, and R. Cavicchioli. 2000. Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtonii. J. Mol. Biol. 297:553-567. [DOI] [PubMed] [Google Scholar]
- 26.Lopez, J. M., and B. Thoms. 1976. Relations between catabolite repression and sporulation in Bacillus subtilis. Arch. Microbiol. 109:181-186. [DOI] [PubMed] [Google Scholar]
- 27.Majumdar, D., Y. J. Avissar, and J. H. Wyche. 1991. Simultaneous and rapid isolation of bacterial and eukaryotic DNA and RNA: a new approach for isolating DNA. BioTechniques 11:94-101. [PubMed] [Google Scholar]
- 28.Mascarenhas, J., M. H. Weber, and P. L. Graumann. 2001. Specific polar localization of ribosomes in Bacillus subtilis depends on active transcription. EMBO Rep. 2:685-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mogk, A., G. Homuth, C. Scholz, L. Kim, F. X. Schmid, and W. Schumann. 1997. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 16:4579-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Völker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schröder, K., P. Graumann, A. Schnuchel, T. A. Holak, and M. A. Marahiel. 1995. Mutational analysis of the putative nucleic acid-binding surface of the cold-shock domain, CspB, revealed an essential role of aromatic and basic residues in binding of single-stranded DNA containing the Y-box motif. Mol. Microbiol. 16:699-708. [DOI] [PubMed] [Google Scholar]
- 32.Serrano, M., S. Hövel, C. P. Moran, Jr., A. O. Henriques, and U. Völker. 2001. Forespore-specific transcription of the lonB gene during sporulation in Bacillus subtilis. J. Bacteriol. 183:2995-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shingler, V. 1996. Signal sensing by sigma 54-dependent regulators: derepression as a control mechanism. Mol. Microbiol. 19:409-416. [DOI] [PubMed] [Google Scholar]
- 34.Stüber, D., H. Matile, and G. Garotta. 1990. System for high-level production in Escherichia coli and rapid purification of recombinant proteins: application to epitope mapping, preparation of antibodies, and structure function analysis, p. 121-152. In I. Lefkovits and B. Pernis (ed.), Immunological methods. Academic Press, Orlando, Fla.
- 35.Suzuki, I., D. A. Los, Y. Kanesaki, K. Mikami, and N. Murata. 2000. The pathway for perception and transduction of low-temperature signals in Synechocystis. EMBO J. 19:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber, M. H. W., C. L. Beckering, and M. A. Marahiel. 2001. Complementation of cold shock proteins by translation initiation factor IF1 in vivo. J. Bacteriol. 183:7381-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber, M. H. W., I. Fricke, N. Doll, and M. A. Marahiel. 2002. CSDBase: an interactive database for cold shock domain-containing proteins and the bacterial cold shock response. Nucleic Acids Res. 30:375-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber, M. H. W., W. Klein, L. Müller, U. M. Niess, and M. A. Marahiel. 2001. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol. Microbiol. 39:1321-1329. [DOI] [PubMed] [Google Scholar]
- 39.Weber, M. H. W., A. V. Volkov, I. Fricke, M. A. Marahiel, and P. L. Graumann. 2001. Localization of cold shock proteins to cytosolic spaces surrounding nucleoids in Bacillus subtilis depends on active transcription. J. Bacteriol. 183:6435-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wistow, G. 1990. Cold shock and DNA binding. Nature 344:823-824. [DOI] [PubMed] [Google Scholar]
- 41.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida, K. I., Y. Fujita, and S. D. Ehrlich. 2000. An operon for a putative ATP-binding cassette transport system involved in acetoin utilization of Bacillus subtilis. J. Bacteriol. 182:5454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]