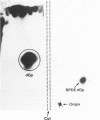
Abstract
Human cancer risk assessment at a genetic level involves the investigation of carcinogen metabolism and DNA adduct formation. Wide interindividual differences in metabolism result in different DNA adduct levels. For this and other reasons, many laboratories have considered DNA adducts to be a measure of the biologically effective dose of a carcinogen. Techniques for studying DNA adducts using chemically specific assays are becoming available. A modification of the 32P-postlabeling assay for polycyclic aromatic hydrocarbon DNA adducts described here provides potential improvements in quantification. DNA adducts, however, reflect only recent exposure to carcinogens; in contrast, genetic testing for metabolic capacity indicates the extent to which carcinogens can be activated and exert genotoxic effects. Such studies may reflect both separate and integrated risk factors together with DNA adduct levels. A recently described restriction fragment length polymorphism for the CYP1A1, which codes for the cytochrome P450 enzyme primarily responsible for the metabolic activation of carcinogenic polycyclic aromatic hydrocarbons, has been found to be associated with lung cancer risk in a Japanese population. In a subset of individuals enrolled in a U.S. lung cancer case-control study, no association with lung cancer was found.
Full text
PDF
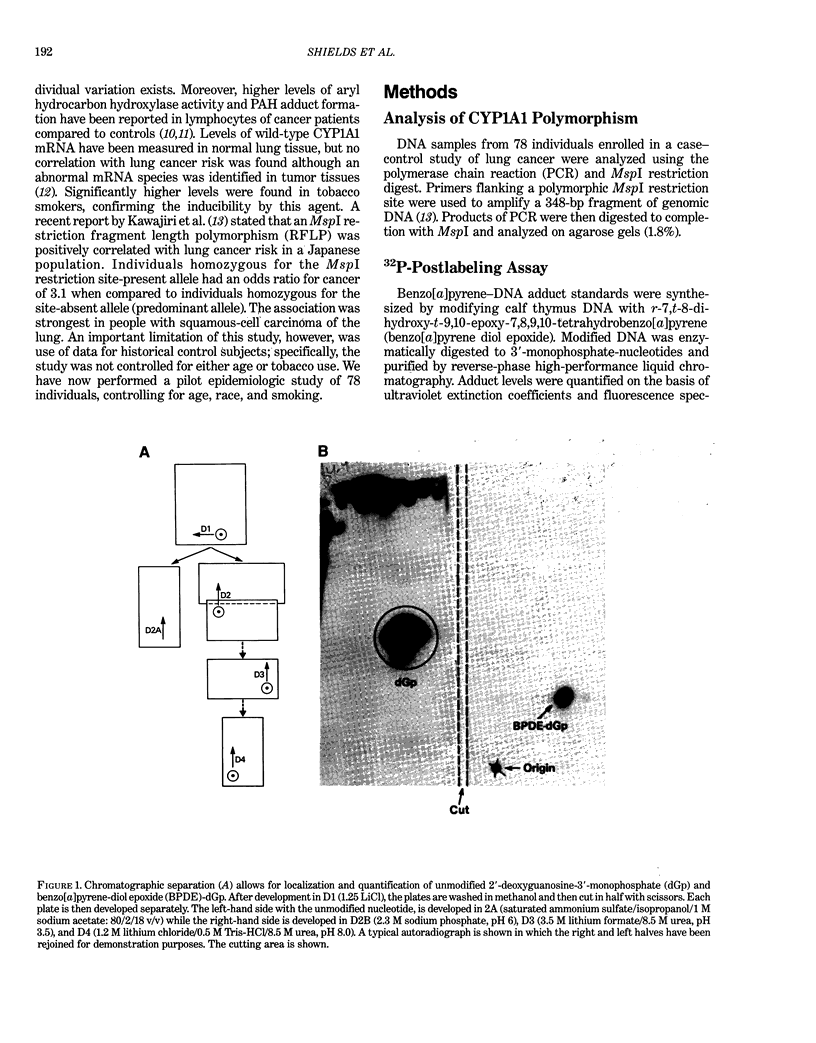
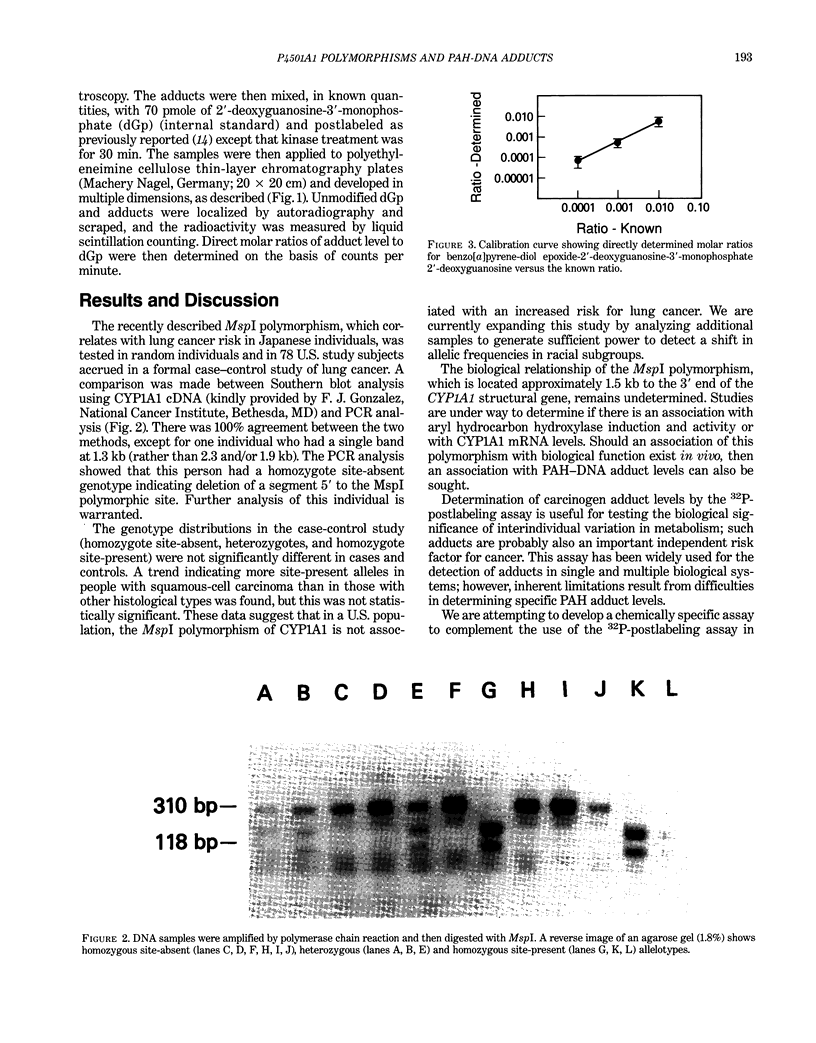

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boles T. C., Hogan M. E. Site-specific carcinogen binding to DNA. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5623–5627. doi: 10.1073/pnas.81.18.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. P., Vedal S., San R. H., Kwan W. F., Nelems B., Enarson D. A., Stich H. F. DNA adducts in bronchial biopsies. Int J Cancer. 1991 Jun 19;48(4):485–492. doi: 10.1002/ijc.2910480403. [DOI] [PubMed] [Google Scholar]
- Gorelick N. J., Wogan G. N. Fluoranthene-DNA adducts: identification and quantification by an HPLC-32P-postlabeling method. Carcinogenesis. 1989 Sep;10(9):1567–1577. doi: 10.1093/carcin/10.9.1567. [DOI] [PubMed] [Google Scholar]
- Hawke L. J., Farrell G. C. Increased binding of benzo[a]pyrene metabolites to lymphocytes from patients with lung cancer. Cancer Lett. 1986 Mar;30(3):289–297. doi: 10.1016/0304-3835(86)90053-4. [DOI] [PubMed] [Google Scholar]
- Jahnke G. D., Thompson C. L., Walker M. P., Gallagher J. E., Lucier G. W., DiAugustine R. P. Multiple DNA adducts in lymphocytes of smokers and nonsmokers determined by 32P-postlabeling analysis. Carcinogenesis. 1990 Feb;11(2):205–211. doi: 10.1093/carcin/11.2.205. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Nakachi K., Imai K., Yoshii A., Shinoda N., Watanabe J. Identification of genetically high risk individuals to lung cancer by DNA polymorphisms of the cytochrome P450IA1 gene. FEBS Lett. 1990 Apr 9;263(1):131–133. doi: 10.1016/0014-5793(90)80721-t. [DOI] [PubMed] [Google Scholar]
- Kouri R. E., McKinney C. E., Slomiany D. J., Snodgrass D. R., Wray N. P., McLemore T. L. Positive correlation between high aryl hydrocarbon hydroxylase activity and primary lung cancer as analyzed in cryopreserved lymphocytes. Cancer Res. 1982 Dec;42(12):5030–5037. [PubMed] [Google Scholar]
- Liou S. H., Jacobson-Kram D., Poirier M. C., Nguyen D., Strickland P. T., Tockman M. S. Biological monitoring of fire fighters: sister chromatid exchange and polycyclic aromatic hydrocarbon-DNA adducts in peripheral blood cells. Cancer Res. 1989 Sep 1;49(17):4929–4935. [PubMed] [Google Scholar]
- Marshall C. J., Vousden K. H., Phillips D. H. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a)pyrene diol-epoxide. Nature. 1984 Aug 16;310(5978):586–589. doi: 10.1038/310586a0. [DOI] [PubMed] [Google Scholar]
- McLemore T. L., Adelberg S., Liu M. C., McMahon N. A., Yu S. J., Hubbard W. C., Czerwinski M., Wood T. G., Storeng R., Lubet R. A. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J Natl Cancer Inst. 1990 Aug 15;82(16):1333–1339. doi: 10.1093/jnci/82.16.1333. [DOI] [PubMed] [Google Scholar]
- Perera F. P., Hemminki K., Young T. L., Brenner D., Kelly G., Santella R. M. Detection of polycyclic aromatic hydrocarbon-DNA adducts in white blood cells of foundry workers. Cancer Res. 1988 Apr 15;48(8):2288–2291. [PubMed] [Google Scholar]
- Phillips D. H. Fifty years of benzo(a)pyrene. Nature. 1983 Jun 9;303(5917):468–472. doi: 10.1038/303468a0. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Hewer A., Martin C. N., Garner R. C., King M. M. Correlation of DNA adduct levels in human lung with cigarette smoking. Nature. 1988 Dec 22;336(6201):790–792. doi: 10.1038/336790a0. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields P. G., Povey A. C., Wilson V. L., Weston A., Harris C. C. Combined high-performance liquid chromatography/32P-postlabeling assay of N7-methyldeoxyguanosine. Cancer Res. 1990 Oct 15;50(20):6580–6584. [PubMed] [Google Scholar]
- Wilson V. L., Basu A. K., Essigmann J. M., Smith R. A., Harris C. C. O6-alkyldeoxyguanosine detection by 32P-postlabeling and nucleotide chromatographic analysis. Cancer Res. 1988 Apr 15;48(8):2156–2161. [PubMed] [Google Scholar]