Abstract
Cigarette smoking is the strongest risk factor for lung cancer, but genetically determined variations in the activities of pulmonary enzyme that metabolize tobacco-derived carcinogens may affect individual risk. To investigate whether these enzymes (e.g., CYP1A-related) can serve as markers for carcinogen-DNA damage, lung tissue specimens were taken during surgery from middle-aged men with either lung cancer or non-neoplastic lung disease. Phase I [aryl hydrocarbon hydroxylase (AHH), ethoxycoumarin O-deethylase (ECOD)] and phase II (epoxide hydrolase, UDP-glucuronosyltransferase, glutathione S-transferase) enzyme activities, glutathione and malondialdehyde contents were determined in lung parenchyma and/or bronchial tissues; some samples were also analyzed for DNA adducts, using 32P-postlabeling. The data were then analyzed for the following: a) differences in metabolic profiles between bronchial and parenchymal lung tissue; b) the effect of recent exposure to tobacco smoke on enzyme inducibility and benzo[a]pyrene metabolism; c) differences in enzyme inducibility between lung cancer and non-lung cancer patients; d) the effect of smoking on metabolism of mutagens in vitro; e) pulmonary DNA adduct levels and AHH activity in lung parenchyma of smokers and ex-smokers; f) lipid peroxidation products in lung tissue from lung cancer and non-lung cancer patients, as related to smoking habits and degree of airway obstruction; and g) prognostic value of AHH pulmonary activity in lung cancer patients. The results demonstrate a pronounced effect of tobacco smoke on pulmonary metabolism of xenobiotics and prooxidant state and suggest the existence of a metabolic phenotype at higher risk for tobacco-associated lung cancer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrov K., Rojas M., Goldberg M., Camus A. M., Bartsch H. A new sensitive fluorometric assay for the metabolism of (--)-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by human hair follicles. Carcinogenesis. 1990 Dec;11(12):2157–2161. doi: 10.1093/carcin/11.12.2157. [DOI] [PubMed] [Google Scholar]
- Anttila S., Hietanen E., Vainio H., Camus A. M., Gelboin H. V., Park S. S., Heikkilä L., Karjalainen A., Bartsch H. Smoking and peripheral type of cancer are related to high levels of pulmonary cytochrome P450IA in lung cancer patients. Int J Cancer. 1991 Mar 12;47(5):681–685. doi: 10.1002/ijc.2910470509. [DOI] [PubMed] [Google Scholar]
- Ayesh R., Idle J. R., Ritchie J. C., Crothers M. J., Hetzel M. R. Metabolic oxidation phenotypes as markers for susceptibility to lung cancer. Nature. 1984 Nov 8;312(5990):169–170. doi: 10.1038/312169a0. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Hietanen E., Petruzzelli S., Giuntini C., Saracci R., Mussi A., Angeletti C. A. Possible prognostic value of pulmonary AH-locus-linked enzymes in patients with tobacco-related lung cancer. Int J Cancer. 1990 Aug 15;46(2):185–188. doi: 10.1002/ijc.2910460207. [DOI] [PubMed] [Google Scholar]
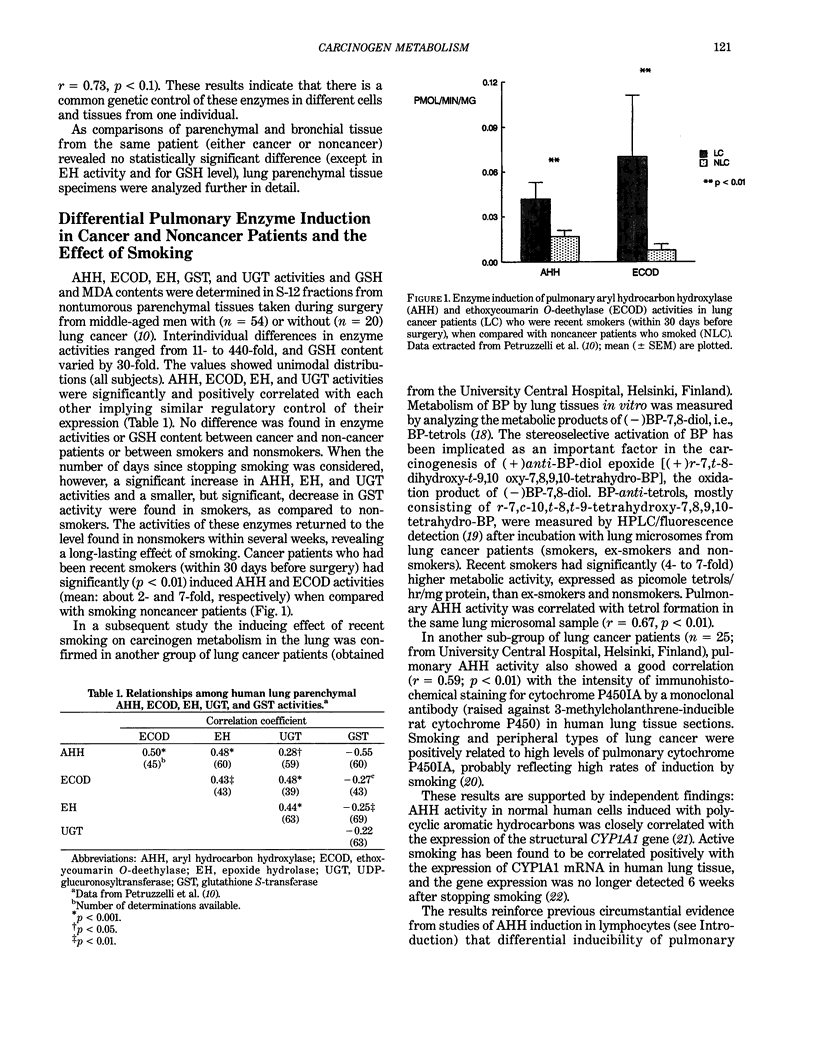
- Bartsch H., Petruzzelli S., De Flora S., Hietanen E., Camus A. M., Castegnaro M., Geneste O., Camoirano A., Saracci R., Giuntini C. Carcinogen metabolism and DNA adducts in human lung tissues as affected by tobacco smoking or metabolic phenotype: a case-control study on lung cancer patients. Mutat Res. 1991 Sep-Oct;250(1-2):103–114. doi: 10.1016/0027-5107(91)90167-m. [DOI] [PubMed] [Google Scholar]
- Caporaso N. E., Tucker M. A., Hoover R. N., Hayes R. B., Pickle L. W., Issaq H. J., Muschik G. M., Green-Gallo L., Buivys D., Aisner S. Lung cancer and the debrisoquine metabolic phenotype. J Natl Cancer Inst. 1990 Aug 1;82(15):1264–1272. doi: 10.1093/jnci/82.15.1264. [DOI] [PubMed] [Google Scholar]
- Church D. F., Pryor W. A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985 Dec;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S., Bennicelli C., Zanacchi P., Camoirano A., Petruzzelli S., Giuntini C. Metabolic activation and deactivation of mutagens by preparations of human lung parenchyma and bronchial tree. Mutat Res. 1984 Jan;139(1):9–14. doi: 10.1016/0165-7992(84)90114-3. [DOI] [PubMed] [Google Scholar]
- De Flora S., Petruzzelli S., Camoirano A., Bennicelli C., Romano M., Rindi M., Ghelarducci L., Giuntini C. Pulmonary metabolism of mutagens and its relationship with lung cancer and smoking habits. Cancer Res. 1987 Sep 1;47(17):4740–4745. [PubMed] [Google Scholar]
- Geneste O., Camus A. M., Castegnaro M., Petruzzelli S., Macchiarini P., Angeletti C. A., Giuntini C., Bartsch H. Comparison of pulmonary DNA adduct levels, measured by 32P-postlabelling and aryl hydrocarbon hydroxylase activity in lung parenchyma of smokers and ex-smokers. Carcinogenesis. 1991 Jul;12(7):1301–1305. doi: 10.1093/carcin/12.7.1301. [DOI] [PubMed] [Google Scholar]
- Gough A. C., Miles J. S., Spurr N. K., Moss J. E., Gaedigk A., Eichelbaum M., Wolf C. R. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature. 1990 Oct 25;347(6295):773–776. doi: 10.1038/347773a0. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human P1-450 gene sequence and correlation of mRNA with genetic differences in benzo[a]pyrene metabolism. Nucleic Acids Res. 1985 Jun 25;13(12):4503–4520. doi: 10.1093/nar/13.12.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri K., Nakachi K., Imai K., Yoshii A., Shinoda N., Watanabe J. Identification of genetically high risk individuals to lung cancer by DNA polymorphisms of the cytochrome P450IA1 gene. FEBS Lett. 1990 Apr 9;263(1):131–133. doi: 10.1016/0014-5793(90)80721-t. [DOI] [PubMed] [Google Scholar]
- Kellermann G., Shaw C. R., Luyten-Kellerman M. Aryl hydrocarbon hydroxylase inducibility and bronchogenic carcinoma. N Engl J Med. 1973 Nov 1;289(18):934–937. doi: 10.1056/NEJM197311012891802. [DOI] [PubMed] [Google Scholar]
- Kouri R. E., McKinney C. E., Slomiany D. J., Snodgrass D. R., Wray N. P., McLemore T. L. Positive correlation between high aryl hydrocarbon hydroxylase activity and primary lung cancer as analyzed in cryopreserved lymphocytes. Cancer Res. 1982 Dec;42(12):5030–5037. [PubMed] [Google Scholar]
- Löfroth G., Rannug A. Ah receptor ligands in tobacco smoke. Toxicol Lett. 1988 Aug;42(2):131–136. doi: 10.1016/0378-4274(88)90070-7. [DOI] [PubMed] [Google Scholar]
- McLemore T. L., Adelberg S., Liu M. C., McMahon N. A., Yu S. J., Hubbard W. C., Czerwinski M., Wood T. G., Storeng R., Lubet R. A. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J Natl Cancer Inst. 1990 Aug 15;82(16):1333–1339. doi: 10.1093/jnci/82.16.1333. [DOI] [PubMed] [Google Scholar]
- Nakachi K., Imai K., Hayashi S., Watanabe J., Kawajiri K. Genetic susceptibility to squamous cell carcinoma of the lung in relation to cigarette smoking dose. Cancer Res. 1991 Oct 1;51(19):5177–5180. [PubMed] [Google Scholar]
- Paoletti P., Pistelli G., Fazzi P., Viegi G., Di Pede F., Giuliano G., Prediletto R., Carrozzi L., Polato R., Saetta M. Reference values for vital capacity and flow-volume curves from a general population study. Bull Eur Physiopathol Respir. 1986 Sep-Oct;22(5):451–459. [PubMed] [Google Scholar]
- Petersen D. D., McKinney C. E., Ikeya K., Smith H. H., Bale A. E., McBride O. W., Nebert D. W. Human CYP1A1 gene: cosegregation of the enzyme inducibility phenotype and an RFLP. Am J Hum Genet. 1991 Apr;48(4):720–725. [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli S., Hietanen E., Bartsch H., Camus A. M., Mussi A., Angeletti C. A., Saracci R., Giuntini C. Pulmonary lipid peroxidation in cigarette smokers and lung cancer patients. Chest. 1990 Oct;98(4):930–935. doi: 10.1378/chest.98.4.930. [DOI] [PubMed] [Google Scholar]
- Poland A., Palen D., Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982 Nov 18;300(5889):271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Pyykkö K., Tuimala R., Aalto L., Perkiö T. Is aryl hydrocarbon hydroxylase activity a new prognostic indicator for breast cancer? Br J Cancer. 1991 Apr;63(4):596–600. doi: 10.1038/bjc.1991.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M., Camus A. M., Alexandrov K., Husgafvel-Pursiainen K., Anttila S., Vainio H., Bartsch H. Stereoselective metabolism of (-)-benzo[a]pyrene-7,8-diol by human lung microsomes and peripheral blood lymphocytes: effect of smoking. Carcinogenesis. 1992 Jun;13(6):929–933. doi: 10.1093/carcin/13.6.929. [DOI] [PubMed] [Google Scholar]
- Sellers T. A., Bailey-Wilson J. E., Elston R. C., Wilson A. F., Elston G. Z., Ooi W. L., Rothschild H. Evidence for mendelian inheritance in the pathogenesis of lung cancer. J Natl Cancer Inst. 1990 Aug 1;82(15):1272–1279. doi: 10.1093/jnci/82.15.1272. [DOI] [PubMed] [Google Scholar]
- Tefre T., Ryberg D., Haugen A., Nebert D. W., Skaug V., Brøgger A., Børresen A. L. Human CYP1A1 (cytochrome P(1)450) gene: lack of association between the Msp I restriction fragment length polymorphism and incidence of lung cancer in a Norwegian population. Pharmacogenetics. 1991 Oct;1(1):20–25. doi: 10.1097/00008571-199110000-00004. [DOI] [PubMed] [Google Scholar]
- Uematsu F., Kikuchi H., Motomiya M., Abe T., Sagami I., Ohmachi T., Wakui A., Kanamaru R., Watanabe M. Association between restriction fragment length polymorphism of the human cytochrome P450IIE1 gene and susceptibility to lung cancer. Jpn J Cancer Res. 1991 Mar;82(3):254–256. doi: 10.1111/j.1349-7006.1991.tb01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


