Abstract
The current generation of biologic markers have three characteristics that differentiate them from previous ones. These include the ability to detect xenobiotics at concentrations at the cellular and molecular level, to detect earlier biologic changes presumptive of disease or disease risk, and to identify a detailed continuum of events between an exposure and resultant disease. If biomarkers are to enhance cancer epidemiology, they must be valid, reliable, and practical. When these characteristics have not been previously demonstrated, pilot studies should be conducted prior to the primary study. Interdisciplinary communication and collaboration is required so that useful markers are selected and that collection and handling, assay, and interpretation are appropriate. The status of many biomarkers is that they have been developed in the laboratory but lack validation for field use. Validation of a marker for use in a population requires attention to issues of background prevalence, sample size, natural history, persistence, variability, confounding factors, and predictive value. Additionally, practical features such as subject preparation, access to specimens, specimen storage aspects, and costs must be clarified. Ultimately, the use of biologic markers in epidemiologic studies will depend on how well the markers increase ability to reduce misclassification, provide for better interpretation of exposure-disease associations, and increase opportunities for prevention. Validation studies and general research using biomarkers also have clinical, ethical, and legal implications. These range from communicating uncertainty about the meaning of a marker to the kinds of societal response that result when groups or individuals are identified as having an "abnormal" marker frequency.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
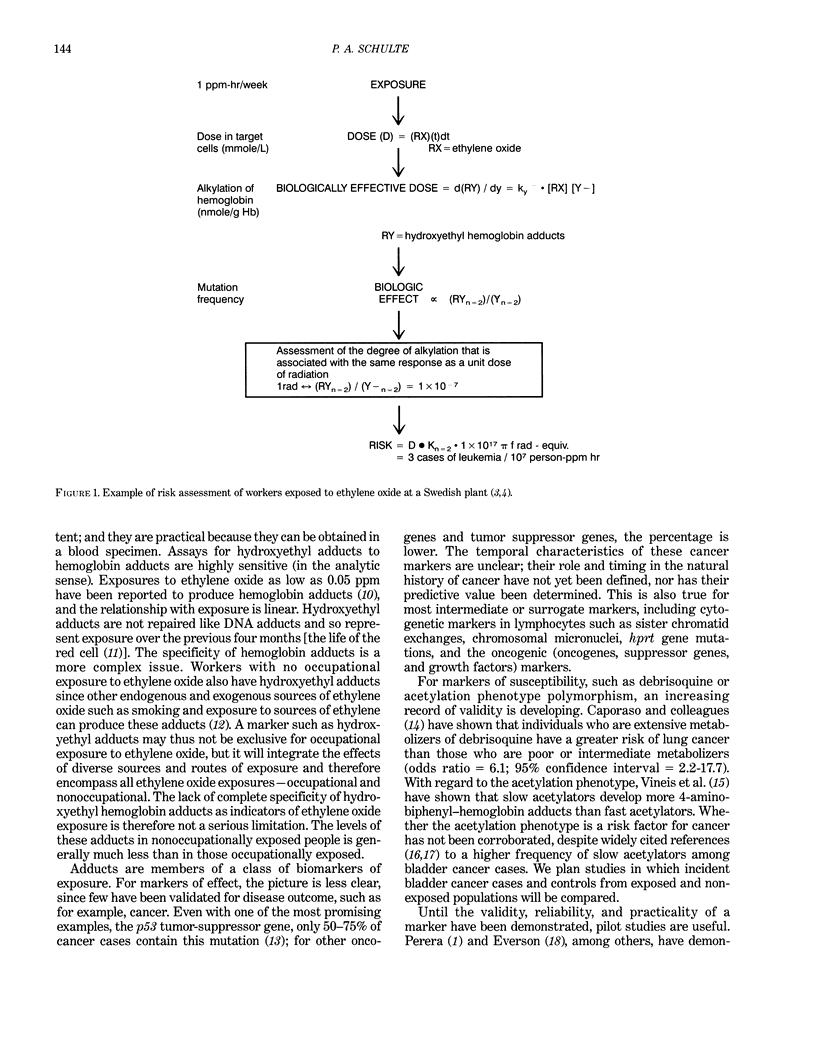
- Calleman C. J., Ehrenberg L., Jansson B., Osterman-Golkar S., Segerbäck D., Svensson K., Wachtmeister C. A. Monitoring and risk assessment by means of alkyl groups in hemoglobin in persons occupationally exposed to ethylene oxide. J Environ Pathol Toxicol. 1978 Nov-Dec;2(2):427–442. [PubMed] [Google Scholar]
- Calleman C. J. Monitoring of background levels of hydroxyethyl adducts in human hemoglobin. Prog Clin Biol Res. 1986;209B:261–270. [PubMed] [Google Scholar]
- Caporaso N. E., Tucker M. A., Hoover R. N., Hayes R. B., Pickle L. W., Issaq H. J., Muschik G. M., Green-Gallo L., Buivys D., Aisner S. Lung cancer and the debrisoquine metabolic phenotype. J Natl Cancer Inst. 1990 Aug 1;82(15):1264–1272. doi: 10.1093/jnci/82.15.1264. [DOI] [PubMed] [Google Scholar]
- Cartwright R. A., Glashan R. W., Rogers H. J., Ahmad R. A., Barham-Hall D., Higgins E., Kahn M. A. Role of N-acetyltransferase phenotypes in bladder carcinogenesis: a pharmacogenetic epidemiological approach to bladder cancer. Lancet. 1982 Oct 16;2(8303):842–845. doi: 10.1016/s0140-6736(82)90810-8. [DOI] [PubMed] [Google Scholar]
- Everson R. B. A review of approaches to the detection of genetic damage in the human fetus. Environ Health Perspect. 1987 Oct;74:109–117. doi: 10.1289/ehp.8774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J., Duncan R. C., Hulka B. S. Biochemical and biological markers: implications for epidemiologic studies. Arch Environ Health. 1989 Nov-Dec;44(6):375–381. doi: 10.1080/00039896.1989.9935910. [DOI] [PubMed] [Google Scholar]
- Osterman-Golkar S., Bergmark E. Occupational exposure to ethylene oxide. Relation between in vivo dose and exposure dose. Scand J Work Environ Health. 1988 Dec;14(6):372–377. doi: 10.5271/sjweh.1906. [DOI] [PubMed] [Google Scholar]
- Perera F. P. Molecular cancer epidemiology: a new tool in cancer prevention. J Natl Cancer Inst. 1987 May;78(5):887–898. [PubMed] [Google Scholar]
- Perera F., Fischman H. K., Hemminki K., Brandt-Rauf P., Niman H. L., Smith S., Toporoff E., O'Dowd K., Tang M. X., Tsai W. Y. Protein binding, sister chromatid exchange and expression of oncogene proteins in patients treated with cisplatinum (cisDDP)-based chemotherapy. Arch Toxicol. 1990;64(5):401–406. doi: 10.1007/BF01973463. [DOI] [PubMed] [Google Scholar]
- Schulte P. A. A conceptual framework for the validation and use of biologic markers. Environ Res. 1989 Apr;48(2):129–144. doi: 10.1016/s0013-9351(89)80029-5. [DOI] [PubMed] [Google Scholar]
- Schulte P. A. Methodologic issues in the use of biologic markers in epidemiologic research. Am J Epidemiol. 1987 Dec;126(6):1006–1016. doi: 10.1093/oxfordjournals.aje.a114740. [DOI] [PubMed] [Google Scholar]
- Schulte P. A., Ringen K., Hemstreet G. P. Optimal management of asymptomatic workers at high risk of bladder cancer. J Occup Med. 1986 Jan;28(1):13–17. doi: 10.1097/00043764-198601000-00006. [DOI] [PubMed] [Google Scholar]
- Skipper P. L., Tannenbaum S. R. Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis. 1990 Apr;11(4):507–518. doi: 10.1093/carcin/11.4.507. [DOI] [PubMed] [Google Scholar]
- Van Sittert N. J., de Jong G., Clare M. G., Davies R., Dean B. J., Wren L. J., Wright A. S. Cytogenetic, immunological, and haematological effects in workers in an ethylene oxide manufacturing plant. Br J Ind Med. 1985 Jan;42(1):19–26. doi: 10.1136/oem.42.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P., Caporaso N. Applications of biochemical epidemiology in the study of human carcinogenesis. Tumori. 1988 Feb 29;74(1):19–26. doi: 10.1177/030089168807400104. [DOI] [PubMed] [Google Scholar]
- Yager J. W., Eastmond D. A., Robertson M. L., Paradisin W. M., Smith M. T. Characterization of micronuclei induced in human lymphocytes by benzene metabolites. Cancer Res. 1990 Jan 15;50(2):393–399. [PubMed] [Google Scholar]


