Abstract
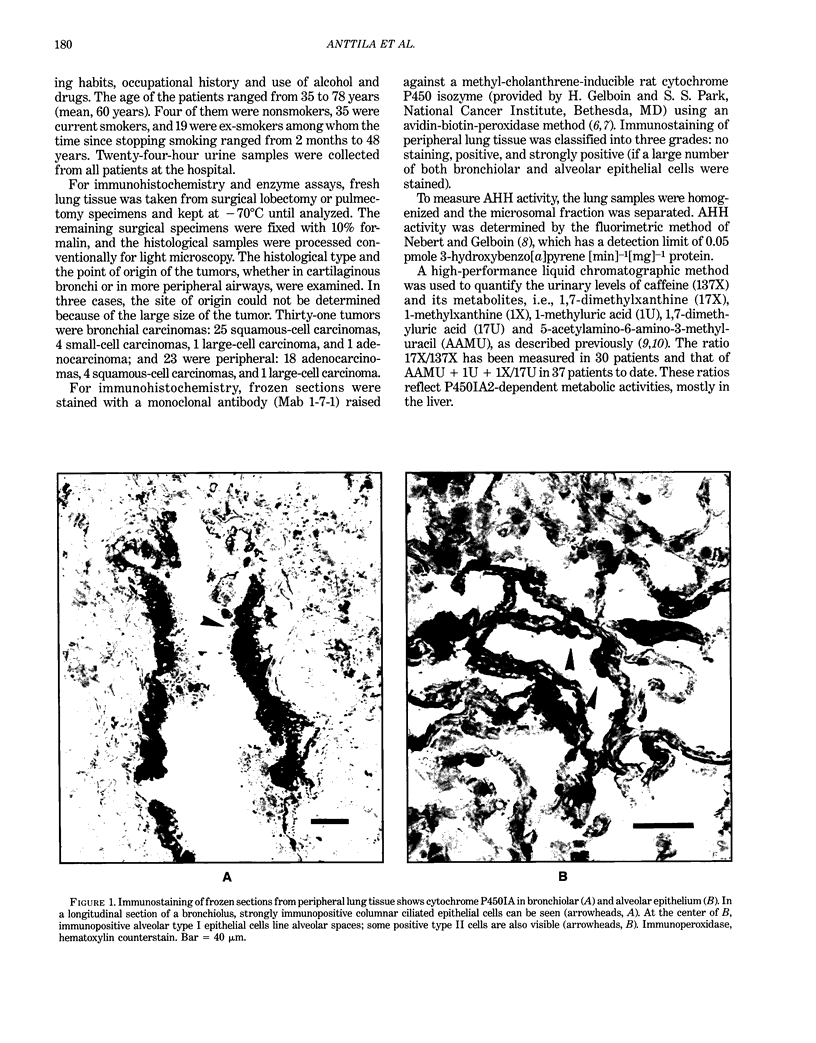
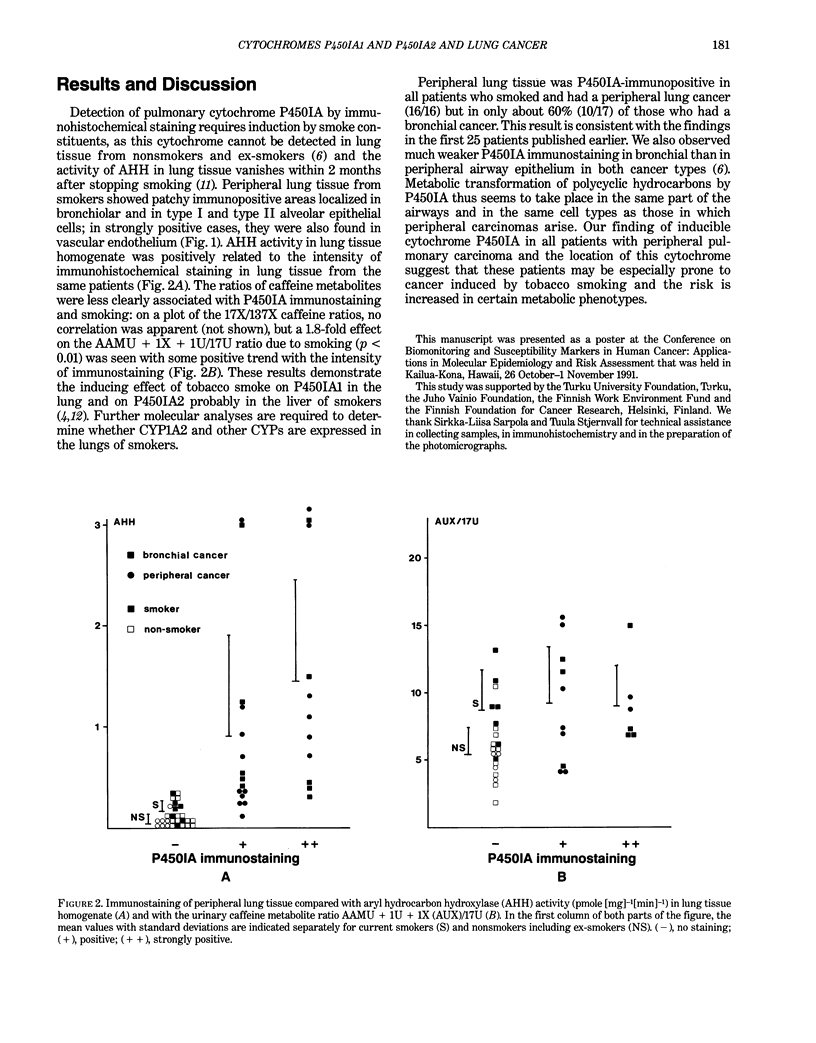
The main polycyclic aromatic hydrocarbon-inducible cytochrome P450 was studied in lung tissue from 57 lung cancer patients by immunohistochemistry, using a monoclonal antibody (1-7-1) that recognizes P450IA1 and P450IA2 isozymes. The intensity of immunostaining was compared with the pulmonary activity of a P450IA1-dependent enzyme, aryl hydrocarbon hydroxylase (AHH), and with P450IA2-related metabolic activity estimated from the ratio of caffeine metabolites in urine. Immunostaining was not observed in peripheral lung tissue of nonsmokers or ex-smokers but was seen in the bronchiolar and alveolar epithelium of all patients who were smokers and had a peripheral carcinoma (16/16) and of 60% (10/17) of those who had a bronchial carcinoma. AHH activity was positively related to the intensity of immunostaining, and an almost 2-fold increase due to smoking was detected in the ratios of caffeine metabolites. These results demonstrate that tobacco smoke induces P450IA1 in the lung and probably P450IA2 in the liver, and suggest a role for certain metabolic phenotypes of P450IA1 in peripheral pulmonary carcinoma.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttila S., Hietanen E., Vainio H., Camus A. M., Gelboin H. V., Park S. S., Heikkilä L., Karjalainen A., Bartsch H. Smoking and peripheral type of cancer are related to high levels of pulmonary cytochrome P450IA in lung cancer patients. Int J Cancer. 1991 Mar 12;47(5):681–685. doi: 10.1002/ijc.2910470509. [DOI] [PubMed] [Google Scholar]
- Berthou F., Ratanasavanh D., Alix D., Carlhant D., Riche C., Guillouzo A. Caffeine and theophylline metabolism in newborn and adult human hepatocytes; comparison with adult rat hepatocytes. Biochem Pharmacol. 1988 Oct 1;37(19):3691–3700. doi: 10.1016/0006-2952(88)90402-9. [DOI] [PubMed] [Google Scholar]
- Butler M. A., Iwasaki M., Guengerich F. P., Kadlubar F. F. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. E., Spielberg S. P., Kalow W. A urinary metabolite ratio that reflects systemic caffeine clearance. Clin Pharmacol Ther. 1987 Aug;42(2):157–165. doi: 10.1038/clpt.1987.126. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Evolution of the P450 gene superfamily: animal-plant 'warfare', molecular drive and human genetic differences in drug oxidation. Trends Genet. 1990 Jun;6(6):182–186. doi: 10.1016/0168-9525(90)90174-5. [DOI] [PubMed] [Google Scholar]
- McLemore T. L., Adelberg S., Liu M. C., McMahon N. A., Yu S. J., Hubbard W. C., Czerwinski M., Wood T. G., Storeng R., Lubet R. A. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J Natl Cancer Inst. 1990 Aug 15;82(16):1333–1339. doi: 10.1093/jnci/82.16.1333. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. II. Cellular responses during enzyme induction. J Biol Chem. 1968 Dec 10;243(23):6250–6261. [PubMed] [Google Scholar]
- Nebert D. W. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol. 1989;20(3):153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- Park S. S., Fujino T., West D., Guengerich F. P., Gelboin H. V. Monoclonal antibodies that inhibit enzyme activity of 3-methylcholanthrene-induced cytochrome P-450. Cancer Res. 1982 May;42(5):1798–1808. [PubMed] [Google Scholar]
- Petruzzelli S., Camus A. M., Carrozzi L., Ghelarducci L., Rindi M., Menconi G., Angeletti C. A., Ahotupa M., Hietanen E., Aitio A. Long-lasting effects of tobacco smoking on pulmonary drug-metabolizing enzymes: a case-control study on lung cancer patients. Cancer Res. 1988 Aug 15;48(16):4695–4700. [PubMed] [Google Scholar]