Abstract
Bacitracin resistance is normally conferred by either of two major mechanisms, the BcrABC transporter, which pumps out bacitracin, or BacA, an undecaprenol kinase that provides C55-isoprenyl phosphate by de novo synthesis. We demonstrate that the Bacillus subtilis bcrC (ywoA) gene, encoding a putative bacitracin transport permease, is an important bacitracin resistance determinant. A bcrC mutant strain had an eightfold-higher sensitivity to bacitracin. Expression of bcrC initiated from a single promoter site that could be recognized by either of two extracytoplasmic function (ECF) σ factors, σX or σM. Bacitracin induced expression of bcrC, and this induction was dependent on σM but not on σX. Under inducing conditions, expression was primarily dependent on σM. As a consequence, a sigM mutant was fourfold more sensitive to bacitracin, while the sigX mutant was only slightly sensitive. A sigX sigM double mutant was similar to a bcrC mutant in sensitivity. These results support the suggestion that one function of B. subtilis ECF σ factors is to coordinate antibiotic stress responses.
Bacitracin, a mixture of related cyclic peptide antibiotics, was isolated almost 60 years ago from a type of “growth-antagonistic” Bacillus strain from the Presbyterian Hospital of New York (15). Bacitracin is produced by some strains of Bacillus licheniformis and Bacillus subtilis and functions as an inhibitor of cell wall biosynthesis (1, 14). By binding to the C55-isoprenyl pyrophosphate in the presence of divalent cations (most efficiently with Zn2+), bacitracin prevents C55-isoprenyl pyrophosphate dephosphorylation and thus interrupts the recycling of C55-isoprenyl pyrophosphate to C55-isoprenyl phosphate (26, 27). C55-isoprenyl pyrophosphate functions as a lipid carrier for the transport across the membrane of the disaccharide-pentapeptide subunits of the peptidoglycan cell wall.
Bacitracin is a potent antibiotic used clinically in combination with other antimicrobial drugs. Two major mechanisms of bacitracin resistance have been studied, the BcrABC transporter, which pumps out bacitracin (20), and BacA, an undecaprenol kinase that generates C55-isoprenyl phosphate by de novo synthesis (2). The BcrABC transporter provides immunity to the bacitracin-producing strain B. licheniformis. In this system, the BcrB and BcrC proteins form a transmembrane channel, while two BcrA proteins function as ATPases to provide energy for transport (19). Recently, the two-component regulatory system BacRS was identified as a regulator of bcrABC expression (18).
The bacitracin resistance gene bacA was first identified in Escherichia coli (2). BacA homologs were also identified in Streptococcus pneumoniae and Staphylococcus aureus and are important for resistance to bacitracin, presumably by increasing the synthesis of C55-isoprenyl phosphate (6). In some gram-negative bacteria, mutations that block the synthesis of exopolysaccharides also lead to bacitracin resistance, presumably by increasing the supply of the common C55-isoprenyl phosphate carrier (22).
We are interested in the physiological roles of the seven extracytoplasmic function (ECF) σ factors (8) in Bacillus subtilis that were revealed by sequencing of the genome (16). We have pursued two parallel strategies: identification of target genes for each σ factor, and characterization of stimuli that activate each σ regulon. Previous studies of two of these σ factors, σX and σW, indicated that one function of the ECF σ factors is to coordinate antibiotic stress responses and cell envelope homeostasis (3, 5, 12). For example, σW controls the fosB fosfomycin resistance gene in B. subtilis (3). Studies using DNA microarrays and reporter fusions have revealed that cell wall biosynthesis inhibitors (e.g., vancomycin, d-cycloserine, cephalosporin, and tunicamycin) strongly induce the expression of both sigW and the σW regulon. During the course of these studies, we found that vancomycin also induces σM and several candidate σM-controlled genes as well as increasing expression of two other ECF σ factors, σY and σV (5).
The bcrC (formerly ywoA) gene, identified as a component of the vancomycin stimulon, encodes a putative bacteriocin permease similar to the BcrC component of the bacitracin immunity system of B. licheniformis (21). In this report, we demonstrate that bcrC is important for bacitracin resistance and is controlled by both σX and σM. In addition to vancomycin, expression of bcrC is inducible by bacitracin, and this induction is dependent on σM but not on σX.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All B. subtilis strains used were derivatives of CU1065 (W168 trpC2 attSPβ). The strains HB7007 (sigX::spc), HB0020 (sigW::MLS), HB0031 (sigM::kan), HB7022 (wild type, Px-cat-lacZ), and HB7023 (sigX Px-cat-lacZ) have been described previously (3, 5, 10). E. coli strain DH5α was used for standard cloning procedures.
Bacteria were grown in Luria-Bertani (LB) medium at 37°C with vigorous shaking. Antibiotics were added to the growth medium when appropriate: 100 μg of ampicillin per ml for E. coli; 100 μg of spectinomycin, 10 μg of kanamycin, 8 μg of neomycin, and 1 μg of erythromycin plus 25 μg of lincomycin (MLS [for macrolide-lincomycin-streptogramin B resistance]) per ml for B. subtilis.
Construction of bcrC and sigX sigM mutants.
To construct the bcrC mutant, primers 679 (5′-GCGGAATTCATGTGCTATACGCAGGCA) and 680 (5′-GCGGGATCCGAGGCTGCCCAATACATCT) were used to amplify an internal fragment of the bcrC gene (∼310 bp) from the B. subtilis chromosomal DNA. The PCR fragment was digested with EcoRI and BamHI and cloned into pMUTIN4 (28) to generate plasmid pSL02. The plasmid was transformed into B. subtilis wild-type strain CU1065 and inserted into the bcrC locus through Campbell integration, selecting for MLSr. The generated strain (bcrC::pMUTIN) was designated HB0106.
A sigX sigM double mutant (HB0097) was constructed by transforming chromosomal DNA from HB0031 (sigM::kan) into HB7007 (sigX::spc) and selecting for Kanr and Spcr.
Construction and analysis of bcrC and sigM transcriptional fusions.
The bcrC promoter region was amplified from the B. subtilis chromosomal DNA by PCR using primers 683 (5′-CCAAGCTTCAGAATCCCCCCAGAAA) and 684 (5′-CGGGATCCGTGATGAAGACCAT). The resulting fragment was digested with HindIII and BamHI and cloned into pJPM122 (25) to generate plasmid pMC100 (PbcrC-cat-lacZ). The cloned sequence of the promoter region was verified by DNA sequencing (Cornell DNA sequencing facility). The promoter fusion was introduced into SPβ prophage by a double-crossover event, in which plasmid pMC100 was linearized with ScaI and transformed into B. subtilis strain ZB307A (25) with selection for neomycin resistance. SPβ lysates were prepared by heat induction and used to transduce various recipient strains: CU1065 (wild type), HB7007 (sigX::spc), HB0020 (sigW::MLS), and HB0031 (sigM::kan). The generated strains were designated HB0108 (CU1065, PbcrC-cat-lacZ), HB0109 (sigX PbcrC-cat-lacZ), HB0110 (sigW PbcrC-cat-lacZ), and HB0111 (sigM PbcrC-cat-lacZ).
Primers 331 (5′-CCCAAGCTTGGGTATATTCCATTGTGCCA) and 436 (5′-CGGGATCCCAGTAAGTCTTCAGCAAGATG) were used to amplify the promoter region of sigM. The PM-cat-lacZ fusion was constructed following the procedure described above. The generated strains were designated HB0069 (CU1065, PM-cat-lacZ) and HB0070 (sigM PM-cat-lacZ). Note that the constructed sigM promoter region includes both the σA- and σM-dependent promoter sites.
To analyze gene expression, the β-galactosidase assay was performed by the procedure of Miller (17).
Overproduction of σX and σM in B. subtilis with a xylose-inducible system.
The sigX open reading frame (ORF) was PCR amplified with primers 790 (5′-GCGGGATCCAAGTGAACGGAGGGGTTTCA) and 791 (5′-GCGGAATTCCCATCGTCAGCCGCTTGTAA), and the sigM ORF was amplified with primers 792 (5′-GCGGGATCCTATAACATAGAGGGGAGAA) and 793 (5′-GCGGAATTCTGGTCGCTCATTTCCCCATT). The two resulting fragments were digested with BamHI and EcoRI and cloned into pXT (a derivative of pDG1731 in which the cloned gene is controlled by a xylose-inducible promoter, PxylA [T. Msadek, personal communication]). The generated plasmids were designated pRA01 (PxylA-sigX) and pRA02 (PxylA-sigM), respectively. The cloned sequences were verified by DNA sequencing (Cornell DNA sequencing facility).
pRA01 and pRA02 were linearized with ScaI and transformed into B. subtilis strain CU1065 with selection for Spcr. The transformants were screened for MLSs and threonine auxotrophy. The resulting strains were designated HB0150 (CU1065, with PxylA-sigX at the thrC locus) and HB0151 (CU1065, PxylA-sigM at the thrC locus). For overproduction of σX and σM, 50% xylose solution was added to the growth medium to a final concentration of 1%.
Bacitracin MIC assay.
Overnight cultures were diluted 1:100 into fresh LB medium in the presence of serial twofold dilutions of bacitracin. After incubation at 37°C for 5 h with shaking, the optical density at 600 nm (OD600)was measured. Bacitracin zinc salt (70,000 U/g) was purchased from Sigma.
Primer extension assay.
RNA was prepared from mid-logarithmic-phase cells (OD600 ≈ 0.4) with the Qiagen RNeasy mini kit. Then 100 μg of total RNA and 2 pmol of end-labeled reverse primer 684 were mixed for each primer extension experiment following the procedures described previously (10). The PCR-amplified bcrC promoter region (with primers 683 and 684) was sequenced with the same end-labeled reverse primer 684, and the reaction products were electrophoresed adjacent to the primer extension products.
Probe preparation and RNA slot blot analysis.
A PCR fragment containing the sigX ORF (described above) was digested with MseI and purified with the Qiagen PCR purification kit. The resulting ∼440-bp fragment (which was deleted in the sigX mutant) was labeled by the 3′ fill-in method with Klenow fragment (3′→ 5′ exonuclease negative) (New England Biolabs), dTTP, and [α-32P]dATP (New England Nuclear; 3,000 Ci/mmol, 10 mCi/μl). The PCR fragment containing the sigM ORF (described above) was digested with SacI and ClaI and purified. The resulting ∼320-bp fragment (which was deleted in the sigM mutant) was labeled by filling in with dCTP and [α-32P]dGTP (New England Nuclear; 3,000 Ci/mmol, 10 mCi/μl).
Primers 683 and 691 (5′-AAGAATTCGAAGAAAACAAGAGAT) were used to amplify the complete bcrC gene, and primers 689 (5′-AAGGATCCCGTTATGTAAAAA) and 690 (5′-CAGAATTCCTCTTGAATTGACAGA) were used to amplify the complete yubB gene. Probe bcrC was labeled by digestion with HindIII, followed by filling in with [α-32P]dATP, and probe yubB was labeled by digestion with BamHI, followed by filling in with dGTP and [α-32P]dATP.
Total RNA was prepared from 3 ml of B. subtilis cell culture (from four individual strains, CU1065, HB7007, HB0031, and HB0097) with and without bacitracin treatment. Bacitracin was added to the cell cultures to a final concentration of 7 U when the OD600 reached 0.4, and samples were collected 2, 5, 10, and 30 min after induction by addition of 1 ml of a chilled phenol-ethanol (5:95) mixture and centrifugation at 5,000 rpm for 1.5 min at 4°C. The cell pellets were resuspended in 100 μl of lysis buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 3 mg of lysozyme, and 10 U of RNasin RNase inhibitor [ Promega]) and incubated at 37°C for 5 min. The Qiagen RNeasy mini kit was used to extract total RNA from the cell lysates.
The RNA samples were denatured by dissolution in 250 μl of ice-cold 10 mM NaOH-1 mM EDTA buffer and applied to a Zeta-Probe blotting membrane (Bio-Rad) with the Bio-Dot SF microfiltration apparatus (Bio-Rad). The blotted membrane was prehybridized at 55°C for more than 1 h, and then labeled probe (heated at 95°C for 10 min) was added to the hybridization tube. Hybridization was performed at 55°C overnight. The blot was washed twice with a low-stringency buffer, 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]), at room temperature, followed by two high-stringency washes (0.1× SSPE) at 55°C. The blot was then wrapped in plastic wrap and exposed to a Phosphor screen (Molecular Dynamics). To quantify the signals, a Storm imaging system (Storm 840; Molecular Dynamics) was used.
For qualitative analysis, 1 μg of total RNA from each preparation was applied to each slot. To measure the induction of bcrC and sigM quantitatively, we applied RNA samples prepared from the wild-type strain on the blot in a serial twofold dilution (2, 1, 0.5, and 0.25 μg of RNA). The slot was hybridized with probes sigM and bcrC, respectively, and the resulting signals were quantified with the ImageQuant data analysis software.
RESULTS
Identification of bcrC by ROMA and vancomycin-induction microarray analysis.
We have developed a runoff transcription/macroarray analysis (ROMA) technique to identify promoter regions recognized by different alternative σ factors (4). In this technique, a restriction digest of B. subtilis chromosomal DNA is transcribed in vitro with RNA polymerase core enzyme, with or without supplementation with the σ factor of interest, to generate a radiolabeled population of runoff RNA molecules. Hybridization of this probe RNA with a DNA macroarray (Sigma/GenoSys) identifies genes that are downstream of promoters that are active in vitro.
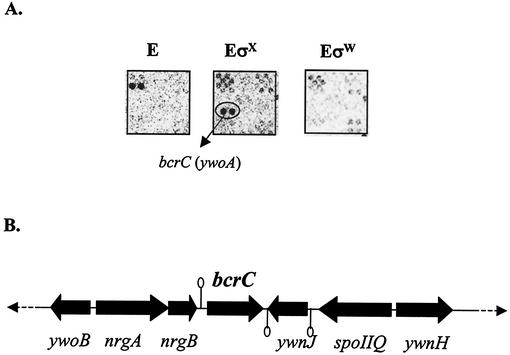
Previously, we reported the use of ROMA to aid in the definition of the B. subtilis σW regulon (4), and we have recently extended these studies to include σX. The ywoA gene was identified as an in vitro target for transcription with the σX holoenzyme but was not found in the RNA samples generated with either core alone or σW holoenzyme (Fig. 1A). In separate studies, we found that ywoA is induced by vancomycin (3.4-fold induction after 3 min of treatment and 5-fold after 10 min of treatment) (5). YwoA is 26% identical to the B. licheniformis bacitracin ABC transporter BcrC subunit. Since ywoA is also important for bacitracin resistance in B. subtilis (see below), we renamed ywoA bcrC.
FIG. 1.
(A) Identification of the bcrC (ywoA) gene by ROMA. Total B. subtilis chromosomal DNA was digested with EcoRI and transcribed in vitro with core alone (E), core with an excess of σX (EσX), or core with an excess of σW (EσW). The bcrC transcript is apparent in experiments with EσX (oval), suggesting that bcrC is a new candidate for the σX regulon. The location of bcrC on the Sigma/GenoSys macroarray is 1G16:4. (B) The bcrC region of the chromosome is illustrated. Predicted rho-independent transcription terminators are indicated by stem-loops.
PbcrC is controlled by two ECF σ factors, σX and σM.
The bcrC gene is located between the nrgAB operon and the convergent ywnJ gene (Fig. 1B) and is therefore likely to be a monocistronic transcription unit. Inspection of the nrgB-bcrC intergenic region identified a candidate promoter element (TGAAACtttt-N13-aGTCta; lowercase indicates less highly conserved bases) similar to those recognized by other characterized B. subtilis ECF σ factors (4, 9, 11-13). It has also been reported that bcrC is a target for σM (A. Moir, personal communication).
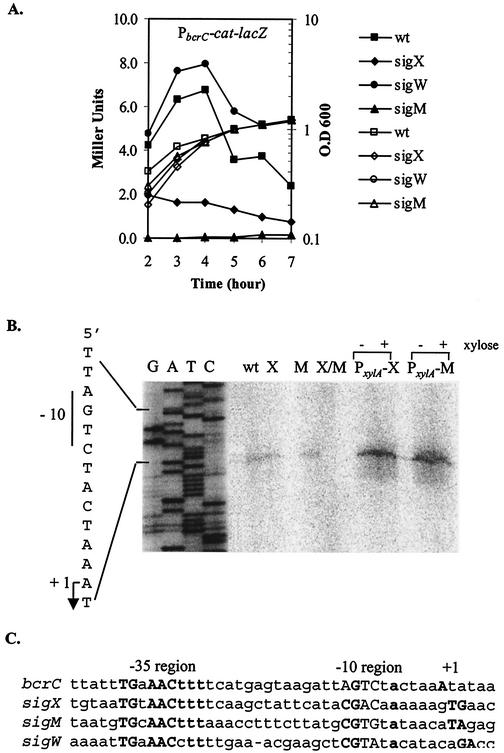
To investigate the roles of σX, σW, and σM in the expression of bcrC, we integrated a PbcrC-cat-lacZ reporter fusion ectopically at SPβ. The resulting reporter fusion was transduced into the wild-type strain CU1065 or mutant strains altered in sigX (HB7007), sigW (HB0020), or sigM (HB0031), and β-galactosidase activity was measured throughout growth. The expression of lacZ was generally very low in the wild-type strain, with maximal expression during late logarithmic growth (Fig. 2A). Consistent with the results of ROMA, expression in the sigX mutant strain was reduced ca. threefold. In contrast, expression was slightly higher than in the wild-type strain in a sigW mutant, consistent with our previous observation that σX and σW are mutually antagonistic (11). We conclude that σX plays a significant role in driving expression from the PbcrC-cat-lacZ reporter fusion.
FIG. 2.
(A) Expression of PbcrC-cat-lacZ during growth in liquid culture. B. subtilis strains HB0108 to HB0111 were grown in LB medium, and β-galactosidase activities were determined at each time point. β-Galactosidase activities are illustrated by solid symbols, and growth curves are illustrated as open symbols. (B) Mapping of the transcription start site by primer extension. RNA was extracted from mid-logarithmic phase wild-type (wt), sigX (X), sigM (M), and sigX sigM (X/M) mutant cells, and the wild-type strain overproducing either σX (PxylA-X) or σM (PxylA-M) in the presence (+) or absence (−) of xylose. The transcription start site and the −10 element are indicated. The same primer was used to sequence the promoter region to index the reverse transcript (lanes AGCT). (C) Comparison of the bcrC promoter sequence with the promoters recognized by the B. subtilis ECF σ factors σX (10), σM (9), and σW (11).
Unexpectedly, however, expression was reduced to background levels in the sigM mutant strain, suggesting that σM also directs transcription from the cloned promoter region and that, in this strain, σX activity at this promoter site was reduced. This is not a general effect on σX activity, since other σX-dependent promoters were still active in the sigM mutant strain (data not shown).
σX and σM initiate transcription of bcrC from the same promoter site.
To further clarify the roles of σX and σM in bcrC transcription, we mapped the bcrC transcriptional start site with primer extension of RNA extracted from the wild type, the sigX mutant, the sigM mutant, and the sigX sigM double mutant strain. Although 100 μg of total RNA was used in each reaction, we observed a very faint transcript only in the wild type, consistent with the low-level expression observed with the reporter fusion. Transcription initiated with an A residue 6 nucleotides downstream from the predicted AGTC −10 element (Fig. 2B). The same transcript was obtained in both the sigX and sigM single mutants but was not detectable in the sigX sigM double mutant. This suggests that either σX or σM can recognize this promoter element in vivo.
To further explore the roles of σX and σM in directing transcription of bcrC, we overproduced either σX or σM with a xylose-inducible promoter system and prepared RNA for primer extension mapping. The bcrC transcript was significantly more abundant in strains containing either the PxylA-sigX or PxylA-sigM fusion (Fig. 2B), even in the absence of xylose induction (presumably due to leaky expression from the xylose-inducible promoter). We did not detect any other transcripts in this assay under conditions that would detect transcripts initiating anywhere within 150 bases upstream of the start codon. Thus, expression of bcrC appears to be dependent on both σX and σM, and these two σ factors activate the same promoter element.
Alignment of the bcrC promoter with the sigX, sigM, and sigW autoregulatory promoters revealed similarities in both the −35 and −10 regions (Fig. 2C). Previously, we demonstrated that σX and σW have overlapping recognition specificity and that −10 elements with a CGTC motif tend to be recognized by both σ factors (23). In this case, both σX and σM recognized an AGTC motif in the −10 region. However, other sequence elements are also likely to participate in promoter discrimination.
bcrC and sigM mutants are sensitive to bacitracin.
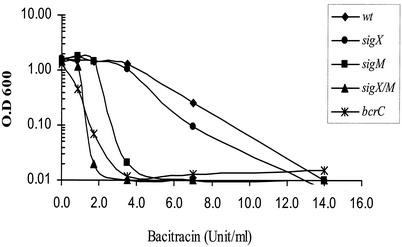
To determine the physiological role of BcrC in B. subtilis, we constructed a bcrC null mutant by gene disruption with an integrational plasmid. The bcrC mutant strain was eightfold more sensitive to bacitracin than the wild-type strain (Fig. 3) but was unaffected in its sensitivity to a variety of other antibiotics (cephalosporin, d-cycloserine, fosfomycin, penicillin, ristocetin, tetracycline, tunicamycin, and vancomycin) and bacteriocins (e.g., bacilysin, colicin E, polymyxin, gramicidin, magainin, and nisin). The sigM mutant had a fourfold-increased sensitivity to bacitracin, while the sigX mutant was only slightly sensitive. The sigX sigM double mutant was as sensitive as the bcrC mutant, consistent with the observation that bcrC expression was eliminated in the sigX sigM double mutant strain.
FIG. 3.
Effects of bacitracin on growth of B. subtilis strains. All strains were grown for 5 h after dilution into LB medium containing the indicated concentration of bacitracin. This experiment was repeated three times, and the results shown are representative. wt, wild type.
Expression of bcrC is induced by bacitracin through σM.
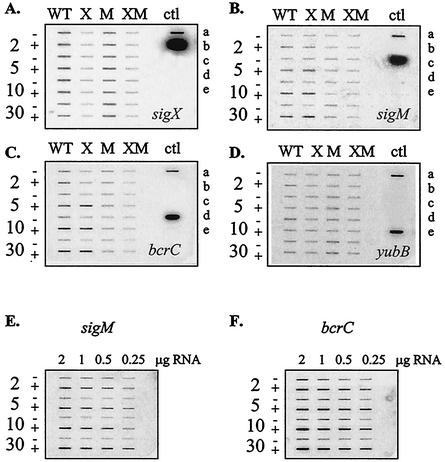
We used slot blot analysis of RNA levels to investigate the ability of bacitracin to induce expression of bcrC, sigX, and sigM (Fig. 4). As expected, the sigX transcript was detected in both wild-type and sigM mutant strains, but only a background level of hybridization was observed in the sigX and sigX sigM mutants. Expression of sigX was not induced by bacitracin (Fig. 4A). In contrast, both sigM (Fig. 4B) and bcrC (Fig. 4C) were induced by bacitracin. Induction was apparent after 2 min of treatment and reached a plateau after 5 min. Quantitation of the RNA signals indicated that sigM was induced about three- to fourfold, while bcrC was induced two- to threefold (Fig. 4E and F; see Materials and Methods also). The induction of bcrC by bacitracin was σM dependent and was readily apparent in both the wild-type and sigX mutant strains but not in the sigM or sigX sigM mutants. We also tested expression of yubB, encoding a possible BacA homolog (see Discussion), and found that this gene was expressed constitutively and was not induced by bacitracin (Fig. 4D).
FIG. 4.
Slot blot analyses of expression of sigX (A), sigM (B), bcrC (C), and yubB (D) in response to bacitracin treatment. WT, X, M, and XM indicate that RNA samples applied to the corresponding column were extracted from the wild-type, sigX, sigM, or sigX sigM mutant strain, respectively. The − and + symbols indicate that RNA samples in this row were extracted from cells not treated (−) or treated (+) with bacitracin after 2, 5, 10 or 30 min. A total of 1 μg of total RNA was applied to each slot in these semiquantitative assays. Positive controls were applied to the control (ctl) column. a, B. subtilis chromosomal DNA; b, PCR fragment of the sigX ORF; c, PCR fragment of the sigM ORF; d, PCR fragment of the bcrC ORF; and e, PCR fragment of the yubB ORF. To quantify the induction of sigM and bcrC expression by bacitracin, RNA samples extracted from the wild-type strain were applied to the blot in a serial twofold dilution series (2, 1, 0.5, and 0.25 μg). The blot was hybridized with probe sigM (E) or bcrC (F), and the resulting signals were quantified with the ImageQuant data analysis software.
Induction of sigM by other antibiotics.
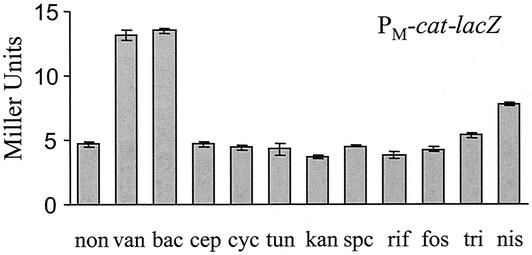
Previously, we reported that sigM and several candidate σM-dependent genes were strongly induced by vancomycin treatment (5), and the results above indicate that sigM was also induced by bacitracin. To test whether other antibiotics could also induce sigM, we treated HB0069 (CU1065 PM-cat-lacZ) with various antibiotics at subinhibitory concentrations and measured lacZ expression by β-galactosidase assays. As expected, both vancomycin and bacitracin induced PM-cat-lacZ expression by more than twofold. Expression of PM-cat-lacZ decreased to background levels in the sigM mutant, and no induction was observed, consistent with the results from slot blot experiments. There was also a slight induction by nisin (Fig. 5). Although we did not measure induction of PM by cephalosporin, d-cycloserine, or tunicamycin in cells grown in liquid culture, we did observe apparent induction on 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) plates, as judged by the formation of blue halos. This same phenomenon was observed previously in induction of the PW-cat-lacZ fusion by cell wall-active antibiotics (5).
FIG. 5.
Expression of PM-cat-lacZ in response to various antibiotic treatments. B. subtilis strain HB0069 (wild type, PM-cat-lacZ) was grown in LB medium to an OD600 of 0.3. The cell culture was split and treated with antibiotics as indicated for 30 min. Cell pellets were immediately harvested by centrifugation and stored at −80°C. Antibiotics and their final concentrations (in micrograms per milliliter) in the medium were as follows: none (non); vancomycin (van), 1; bacitracin (bac), 100 (=7 U/ml); cephalosporin (cep), 1; d-cycloserine (cyc), 10; tunicamycin (tun), 50; kanamycin (kan), 100; spectinomycin (spc), 100; rifampin (rif), 20; fosfomycin (fos), 100; Triton X-100 (tri), 0.05%; and nisin (nis), 50. Error bars represent standard deviations from the average of three independent experiments.
DISCUSSION
We identified the bcrC (formerly ywoA) gene as a target gene for regulation by ECF σ factors based on two independent lines of evidence. First, bcrC was identified as an in vitro target for σX-directed transcription in studies of the σX regulon with the ROMA technique (Fig. 1A). Second, bcrC was found to be a component of the vancomycin stimulon, which includes numerous members of both the σW and σM regulons (5). The results presented here demonstrate that bcrC is an important determinant of bacitracin resistance in B. subtilis and that its expression is controlled by both σX and σM. Induction of bcrC by bacitracin (Fig. 4C) and vancomycin (5) was dependent on σM and independent of σX (Fig. 4C and data not shown).
Judging from the similarity to the BcrABC efflux system, we hypothesize that BcrC may form a dimeric channel that facilitates the efflux of bacitracin. The protein(s) that may function to energize such an efflux pump (analogous to BcrA) is currently not known. Similarly, E. coli encodes a BcrC homolog but does not encode obvious homologs of BcrA or BcrB, suggesting that BcrC may interact heterologously with another ABC transporter system to derive the energy to support active export (7). Precedence for such a heterologous interaction is provided by the Staphylococcus epidermidis erythromycin exporter MsrA, an ATP-binding protein which interacts with transmembrane proteins from S. aureus and Staphylococcus (24). Recently, Ohki and colleagues also reported that a ywoA mutant was hypersensitive to bacitracin (R. Ohki et al., Abstr. 102nd Annu. Meet. Am. Soc. Microbiol., abstr. 240, 2002). Furthermore, they reported that disruption of several genes encoding putative ATP-binding proteins (e.g., yxlF, yfiL, yhcH, and ycbN) did not have any effect on bacitracin resistance.
The bacitracin resistance gene bacA was first identified in E. coli and encodes a membrane-associated isoprenol kinase (2). BacA, which functions in the de novo synthesis of C55-isoprenyl phosphate, also confers bacitracin resistance in S. pneumoniae and S. aureus (6). B. subtilis also encodes a BacA homolog: the product of the yubB gene is 44% identical to E. coli BacA. To investigate the possible role of YubB in bacitracin resistance, we sought to disrupt the gene by allelic replacement. However, several attempts were unsuccessful, indicating that the gene may be essential. Expression of yubB was not induced by bacitracin, nor was it affected by mutations in sigX, sigM, or both genes (Fig. 4D). Thus, the possible role of YubB in bacitracin resistance remains an open question.
The results presented here further strengthen the functional link between ECF σ factors and resistance to antimicrobial compounds. Previously, we demonstrated that the σW regulon is strongly induced by vancomycin and other inhibitors of cell wall biosynthesis (5). At least one member of the σW regulon, FosB, plays a direct role in the detoxification of an antibiotic (3), and others may also contribute to antibiotic resistance (12). The σX regulon has been found to include both the pssA-psd operon and the dlt operon. The products of these operons both serve to decrease the net negative charge density in the cell envelope, and this contributes to increased resistance to cationic antimicrobial peptides (M. Cao and J. D. Helmann, unpublished results).
The discovery of σM and the σM regulon as a component of the vancomycin stimulon (5) and the finding that bcrC contributes to bacitracin resistance suggest that this σ factor also plays a significant role in antibiotic resistance. The functions of the remaining four ECF σ factors encoded in B. subtilis (σV, σY, σZ, and σYlaC) are still unknown.
Acknowledgments
We thank Rania Abou-Kandil for construction of the PxylA-sigX and PxylA-sigM constructs used in this work, Suping Liu for construction of the bcrC::pMUTIN mutant strain, and T. Msadek for providing plasmid pXT.
This work was supported by NIH grant GM-47446 (to J.D.H.).
REFERENCES
- 1.Azevedo, E. C., E. M. Rios, K. Fukushima, and G. M. Campos-Takaki. 1993. Bacitracin production by a new strain of Bacillus subtilis. Extraction, purification, and characterization. Appl. Biochem. Biotechnol. 42:1-7. [DOI] [PubMed] [Google Scholar]
- 2.Cain, B. D., P. J. Norton, W. Eubanks, H. S. Nick, and C. M. Allen. 1993. Amplification of the bacA gene confers bacitracin resistance to Escherichia coli. J. Bacteriol. 175:3784-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, M., B. A. Bernat, Z. Wang, R. N. Armstrong, and J. D. Helmann. 2001. FosB, a cysteine-dependent fosfomycin resistance protein under the control of σW, an extracytoplasmic-function sigma factor in Bacillus subtilis. J. Bacteriol. 183:2380-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, M., P. A. Kobel, M. M. Morshedi, M. F. Wu, C. Paddon, and J. D. Helmann. 2002. Defining the Bacillus subtilis σW regulon: a comparative analysis of promoter consensus search, run-off transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J. Mol. Biol. 316:443-457. [DOI] [PubMed] [Google Scholar]
- 5.Cao, M., T. Wang, R. Ye, and J. D. Helmann. 2002. Antibiotics that inhibit cell wall biosynthesis induce expression of the Bacillus subtilis σW and σM regulons. Mol. Microbiol. 45:1267-1276. [DOI] [PubMed]
- 6.Chalker, A. F., K. A. Ingraham, R. D. Lunsford, A. P. Bryant, J. Bryant, N. G. Wallis, J. P. Broskey, S. C. Pearson, and D. J. Holmes. 2000. The bacA gene, which determines bacitracin susceptibility in Streptococcus pneumoniae and Staphylococcus aureus, is also required for virulence. Microbiology 146:1547-1553. [DOI] [PubMed] [Google Scholar]
- 7.Harel, Y. M., A. Bailone, and E. Bibi. 1999. Resistance to bacitracin as modulated by an Escherichia coli homologue of the bacitracin ABC transporter BcrC subunit from Bacillus licheniformis. J. Bacteriol. 181:6176-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helmann, J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47-110. [DOI] [PubMed] [Google Scholar]
- 9.Horsburgh, M. J., and A. Moir. 1999. Sigma M, an ECF RNA polymerase sigma factor of Bacillus subtilis 168, is essential for growth and survival in high concentrations of salt. Mol. Microbiol. 32:41-50. [DOI] [PubMed] [Google Scholar]
- 10.Huang, X., A. Decatur, A. Sorokin, and J. D. Helmann. 1997. The Bacillus subtilis σX protein is an extracytoplasmic function sigma factor contributing to the survival of high-temperature stress. J. Bacteriol. 179:2915-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, X., K. L. Fredrick, and J. D. Helmann. 1998. Promoter recognition by Bacillus subtilis σW: autoregulation and partial overlap with the σX regulon. J. Bacteriol. 180:3765-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, X., A. Gaballa, M. Cao, and J. D. Helmann. 1999. Identification of target promoters for the Bacillus subtilis extracytoplasmic function σ factor σW. Mol. Microbiol. 31:361-371. [DOI] [PubMed] [Google Scholar]
- 13.Huang, X., and J. D. Helmann. 1998. Identification of target promoters for the Bacillus subtilis σX factor with a consensus-directed search. J. Mol. Biol. 279:165-173. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara, H., M. Takoh, R. Nishibayashi, and A. Sato. 2002. Distribution and variation of bacitracin synthetase gene sequences in laboratory stock strains of Bacillus licheniformis. Curr. Microbiol. 45:18-23. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, B. A., H. Anker, and F. L. Meleney. 1945. Bacitracin: a new antibiotic produced by a member of the B. subtilis group. Science 102:376-377. [DOI] [PubMed] [Google Scholar]
- 16.Kunst, F., N. Ogasawara, I. Moszer, et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 17.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Neumuller, A. M., D. Konz, and M. A. Marahiel. 2001. The two-component regulatory system BacRS is associated with bacitracin ‘self-resistance' of Bacillus licheniformis ATCC 10716. Eur. J. Biochem. 268:3180-3189. [DOI] [PubMed] [Google Scholar]
- 19.Podlesek, Z., A. Comino, B. Herzog-Velikonja, and M. Grabnar. 2000. The role of the bacitracin ABC transporter in bacitracin resistance and collateral detergent sensitivity. FEMS Microbiol. Lett. 188:103-106. [DOI] [PubMed] [Google Scholar]
- 20.Podlesek, Z., A. Comino, B. Herzog-Velikonja, D. Zgur-Bertok, R. Komel, and M. Grabnar. 1995. Bacillus licheniformis bacitracin-resistance ABC transporter: relationship to mammalian multidrug resistance. Mol. Microbiol. 16:969-976. [DOI] [PubMed] [Google Scholar]
- 21.Podlesek, Z., B. Herzog, and A. Comino. 1997. Amplification of bacitracin transporter genes in the bacitracin producing Bacillus licheniformis. FEMS Microbiol. Lett. 157:201-205. [DOI] [PubMed] [Google Scholar]
- 22.Pollock, T. J., L. Thorne, M. Yamazaki, M. J. Mikolajczak, and R. W. Armentrout. 1994. Mechanism of bacitracin resistance in gram-negative bacteria that synthesize exopolysaccharides. J. Bacteriol. 176:6229-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu, J., and J. D. Helmann. 2001. The −10 region is a key promoter specificity determinant for the Bacillus subtilis extracytoplasmic function sigma factors σX and σW. J. Bacteriol. 183:1921-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross, J. I., E. A. Eady, J. H. Cove, and S. Baumberg. 1995. Identification of a chromosomally encoded ABC-transport system with which the staphylococcal erythromycin exporter MsrA may interact. Gene 153:93-98. [DOI] [PubMed] [Google Scholar]
- 25.Slack, F. J., J. P. Mueller, and A. L. Sonenshein. 1993. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J. Bacteriol. 175:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone, K. J., and J. L. Strominger. 1971. Mechanism of action of bacitracin: complexation with metal ion and C55-isoprenyl pyrophosphate. Proc. Natl. Acad. Sci. USA 68:3223-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storm, D. R., and J. L. Strominger. 1973. Complex formation between bacitracin peptides and isoprenyl pyrophosphates. The specificity of lipid-peptide interactions. J. Biol. Chem. 248:3940-3945. [PubMed] [Google Scholar]
- 28.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]