Abstract
Several genes involved in the metabolism of carcinogens have been found to be polymorphic in human populations and are associated with increased risk of cancer at some sites. This study focuses on the polymorphic enzyme glutathione transferase mu (GT mu). Smokers with low lymphocyte GT mu activity are at an approximately 2-fold higher risk for lung cancer and an approximately 3-fold higher risk for stomach and colon adenocarcinomas. Recent cloning and sequencing of the GST1 gene has allowed the development of convenient genotyping methods based on restriction fragment length polymorphisms (RFLP) or the polymerase chain reaction (PCR). The GST1 polymorphism has been shown to be a deletion of the gene locus. To detect the presence or absence of the gene we amplified exons 4-5 and/or exons 6-7 of the GST1 gene by PCR. PCR amplification produced bands of 215-bp or 273-bp from individuals with one or two copies of the GST1 allele and no band if the individual was homozygously deleted (0/0). In the exon 6-7 PCR, we co-amplified a 268-bp portion of the beta-globin gene as an internal reference standard for quantitative analysis of product yield. This allowed homozygote individuals (+/+) to be distinguished from heterozygotes (+/0). We have compared the GST1 genotype to lymphocyte GT mu activity measured on trans-stilbene oxide (TSO) in the lymphocytes of 45 individuals. Low GT mu activity (< 67 pmole/min/10(7) cells) was strongly associated (24/24) with the GST1 0/0 genotype. With the exception of one individual, activities greater than 67 pmole/min/10(7) were associated with the presence of the GST1 allele (20/21). Individuals with the highest GT-TSO activity were found to be homozygous for GST1. (+/+), while heterozygotes (+/0) generally had lower activity, suggesting a gene dosage effect in lymphocytes.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

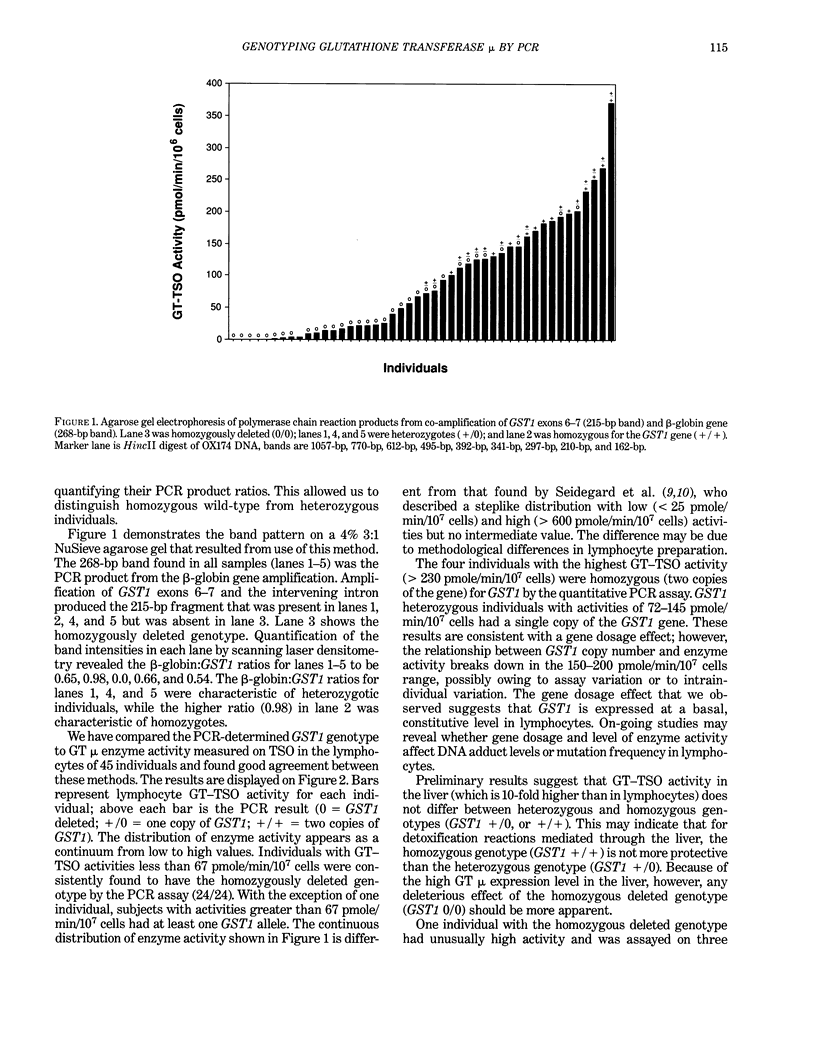
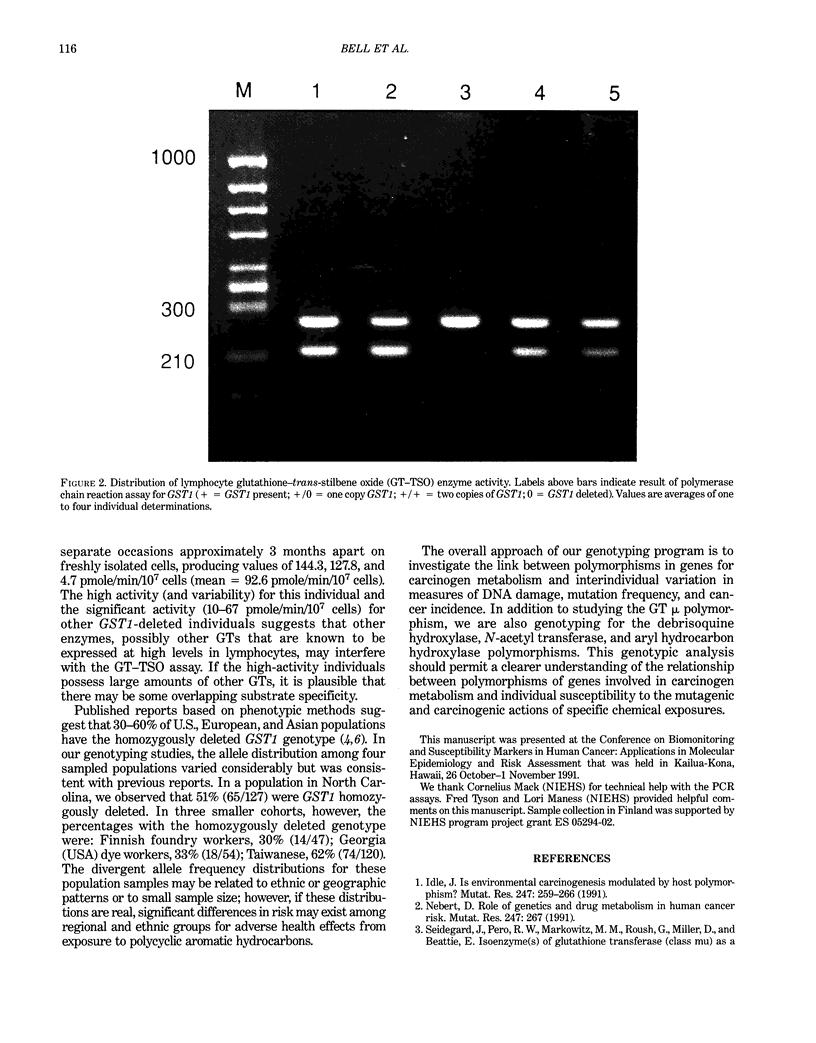

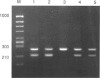
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell E., Takahashi Y., Abramovitz M., Peretz M., Listowsky I. A distinct human testis and brain mu-class glutathione S-transferase. Molecular cloning and characterization of a form present even in individuals lacking hepatic type mu isoenzymes. J Biol Chem. 1990 Jun 5;265(16):9188–9193. [PubMed] [Google Scholar]
- Comstock K. E., Sanderson B. J., Claflin G., Henner W. D. GST1 gene deletion determined by polymerase chain reaction. Nucleic Acids Res. 1990 Jun 25;18(12):3670–3670. doi: 10.1093/nar/18.12.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idle J. R. Is environmental carcinogenesis modulated by host polymorphism? Mutat Res. 1991 Apr;247(2):259–266. doi: 10.1016/0027-5107(91)90021-f. [DOI] [PubMed] [Google Scholar]
- Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat Res. 1988 Dec;202(2):343–361. doi: 10.1016/0027-5107(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Liu Y. H., Taylor J., Linko P., Lucier G. W., Thompson C. L. Glutathione S-transferase mu in human lymphocyte and liver: role in modulating formation of carcinogen-derived DNA adducts. Carcinogenesis. 1991 Dec;12(12):2269–2275. doi: 10.1093/carcin/12.12.2269. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W. Role of genetics and drug metabolism in human cancer risk. Mutat Res. 1991 Apr;247(2):267–281. doi: 10.1016/0027-5107(91)90022-g. [DOI] [PubMed] [Google Scholar]
- Neubauer A., Neubauer B., Liu E. Polymerase chain reaction based assay to detect allelic loss in human DNA: loss of beta-interferon gene in chronic myelogenous leukemia. Nucleic Acids Res. 1990 Feb 25;18(4):993–998. doi: 10.1093/nar/18.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W. The hereditary transmission of high glutathione transferase activity towards trans-stilbene oxide in human mononuclear leukocytes. Hum Genet. 1985;69(1):66–68. doi: 10.1007/BF00295531. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Vorachek W. R., Pero R. W., Pearson W. R. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R. C., Matharoo B., Faulder G. C., Jones P., Cotton W., Elder J. B., Deakin M. The human glutathione S-transferases: a case-control study of the incidence of the GST1 0 phenotype in patients with adenocarcinoma. Carcinogenesis. 1991 Jan;12(1):25–28. doi: 10.1093/carcin/12.1.25. [DOI] [PubMed] [Google Scholar]
- Taylor J. B., Oliver J., Sherrington R., Pemble S. E. Structure of human glutathione S-transferase class Mu genes. Biochem J. 1991 Mar 1;274(Pt 2):587–593. doi: 10.1042/bj2740587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorachek W. R., Pearson W. R., Rule G. S. Cloning, expression, and characterization of a class-mu glutathione transferase from human muscle, the product of the GST4 locus. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4443–4447. doi: 10.1073/pnas.88.10.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]