Abstract
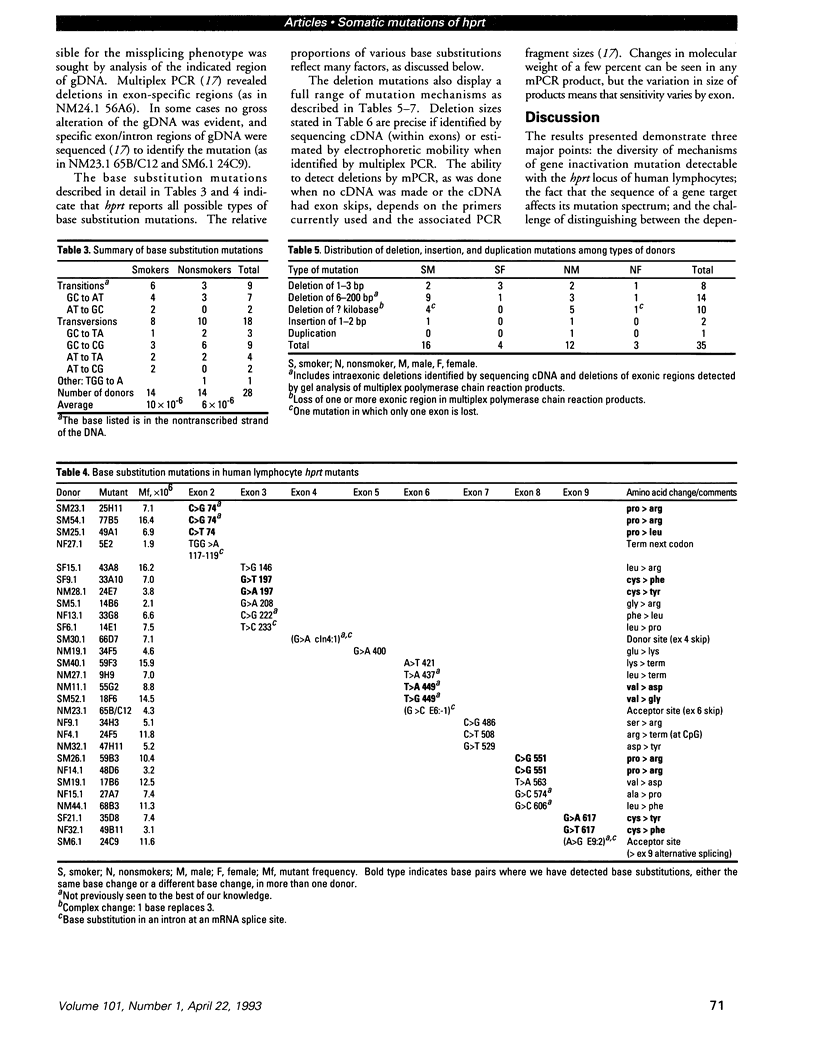
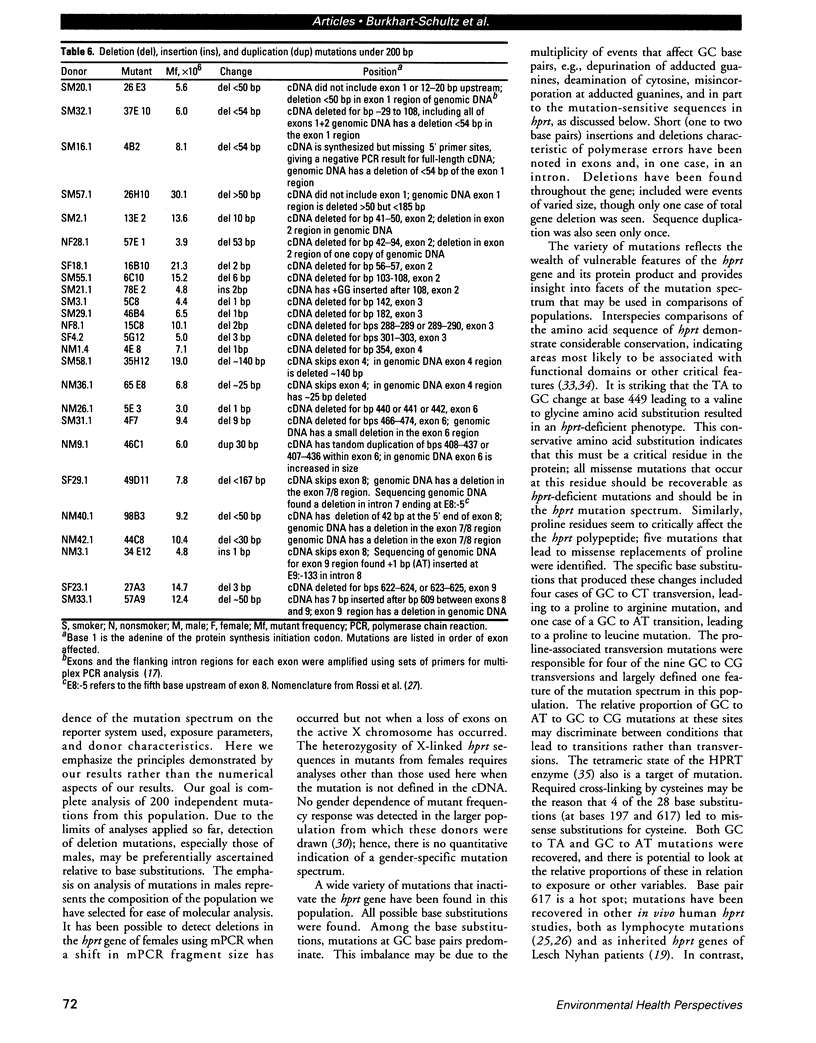
The ability to recognize a change in mutation spectrum after an exposure to a toxic substance and then relate that exposure to health risk depends on the knowledge of mutations that occur in the absence of exposure. Toward this end, we have been studying both the frequency and molecular nature of mutations of the hypoxanthine phosphoribosyltransferase (hprt) gene in peripheral blood lymphocytes as surrogate reporters of genetic damage. We have analyzed mutants, one per donor to ensure independence, from a control population in which the quantitative effects of smoking and age on mutant frequency have been well defined. Analyses of cDNA and genomic DNA by polymerase chain reaction and sequencing have identified the mutations in 63 mutants, 45 from males and 18 from females, of which 34 were smokers and 29 were nonsmokers. Slightly less than half of the mutations were base substitutions; they were predominantly at GC base pairs. Different mutations at the same site indicated that there are features of the hprt polypeptide that affect the mutation spectrum. Two pairs of identical mutations indicated that there may also be hot spots. Mutations not previously reported have been detected, indicating that the mutation spectrum is only partly defined. The remainder of the mutations were deletions or insertions/duplications; deletions ranged from one base pair to complete loss of the locus. Despite a small average increase in mutant frequency for smokers, an increased proportion of base substitutions at AT base pairs in smokers (p = 0.2) hinted at a smoking-associated shift in the mutation spectrum.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., Nicklas J. A., O'Neill J. P., Robison S. H. In vivo somatic mutations in humans: measurement and analysis. Annu Rev Genet. 1990;24:305–326. doi: 10.1146/annurev.ge.24.120190.001513. [DOI] [PubMed] [Google Scholar]
- Bochkarev M. N., Kulbakina N. A., Zhdanova N. S., Rubtsov N. B., Zakian S. M., Serov O. L. Evidence for tetrameric structure of mammalian hypoxanthine phosphoribosyltransferase. Biochem Genet. 1987 Feb;25(1-2):153–160. doi: 10.1007/BF00498958. [DOI] [PubMed] [Google Scholar]
- Bronstein S. M., Skopek T. R., Swenberg J. A. Efficient repair of O6-ethylguanine, but not O4-ethylthymine or O2-ethylthymine, is dependent upon O6-alkylguanine-DNA alkyltransferase and nucleotide excision repair activities in human cells. Cancer Res. 1992 Apr 1;52(7):2008–2011. [PubMed] [Google Scholar]
- Cariello N. F., Craft T. R., Vrieling H., van Zeeland A. A., Adams T., Skopek T. R. Human HPRT mutant database: software for data entry and retrieval. Environ Mol Mutagen. 1992;20(2):81–83. doi: 10.1002/em.2850200202. [DOI] [PubMed] [Google Scholar]
- Cariello N. F., Swenberg J. A., Skopek T. R. In vitro mutational specificity of cisplatin in the human hypoxanthine guanine phosphoribosyltransferase gene. Cancer Res. 1992 May 15;52(10):2866–2873. [PubMed] [Google Scholar]
- Cohen S. M., Ellwein L. B. Genetic errors, cell proliferation, and carcinogenesis. Cancer Res. 1991 Dec 15;51(24):6493–6505. [PubMed] [Google Scholar]
- Davidson B. L., Tarlé S. A., Palella T. D., Kelley W. N. Molecular basis of hypoxanthine-guanine phosphoribosyltransferase deficiency in ten subjects determined by direct sequencing of amplified transcripts. J Clin Invest. 1989 Jul;84(1):342–346. doi: 10.1172/JCI114160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Voss H., Rice P., Civitello A., Stegemann J., Schwager C., Zimmermann J., Erfle H., Caskey C. T., Ansorge W. Automated DNA sequencing of the human HPRT locus. Genomics. 1990 Apr;6(4):593–608. doi: 10.1016/0888-7543(90)90493-e. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., Zimmerman L. J., Harrington-Brock K., Burnette L., Moore M. M., Nicklas J. A., O'Neill J. P., Albertini R. J. V(D)J recombinase-mediated deletion of the hprt gene in T-lymphocytes from adult humans. Mutat Res. 1992 Sep;283(1):13–20. doi: 10.1016/0165-7992(92)90116-y. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., Zimmerman L. J., Lippert M. J., Nicklas J. A., O'Neill J. P., Albertini R. J. V(D)J recombinase-like activity mediates hprt gene deletion in human fetal T-lymphocytes. Cancer Res. 1991 Nov 1;51(21):6001–6005. [PubMed] [Google Scholar]
- Giannelli F., Green P. M., High K. A., Sommer S., Lillicrap D. P., Ludwig M., Olek K., Reitsma P. H., Goossens M., Yoshioka A. Haemophilia B: database of point mutations and short additions and deletions--second edition. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2193–2219. doi: 10.1093/nar/19.suppl.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., Edwards A., Civitello A. B., Caskey C. T. Multiplex DNA deletion detection and exon sequencing of the hypoxanthine phosphoribosyltransferase gene in Lesch-Nyhan families. Genomics. 1990 Jun;7(2):235–244. doi: 10.1016/0888-7543(90)90545-6. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grist S. A., McCarron M., Kutlaca A., Turner D. R., Morley A. A. In vivo human somatic mutation: frequency and spectrum with age. Mutat Res. 1992 Apr;266(2):189–196. doi: 10.1016/0027-5107(92)90186-6. [DOI] [PubMed] [Google Scholar]
- Harris C. C. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res. 1991 Sep 15;51(18 Suppl):5023s–5044s. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Janatipour M., Trainor K. J., Kutlaca R., Bennett G., Hay J., Turner D. R., Morley A. A. Mutations in human lymphocytes studied by an HLA selection system. Mutat Res. 1988 Mar;198(1):221–226. doi: 10.1016/0027-5107(88)90058-9. [DOI] [PubMed] [Google Scholar]
- King A., Melton D. W. Characterisation of cDNA clones for hypoxanthine-guanine phosphoribosyltransferase from the human malarial parasite, Plasmodium falciparum: comparisons to the mammalian gene and protein. Nucleic Acids Res. 1987 Dec 23;15(24):10469–10481. doi: 10.1093/nar/15.24.10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoizumi S., Akiyama M., Hirai Y., Kusunoki Y., Tanabe K., Umeki S. Spontaneous loss and alteration of antigen receptor expression in mature CD4+ T cells. J Exp Med. 1990 Jun 1;171(6):1981–1999. doi: 10.1084/jem.171.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoizumi S., Umeki S., Akiyama M., Hirai Y., Kusunoki Y., Nakamura N., Endoh K., Konishi J., Sasaki M. S., Mori T. Frequency of mutant T lymphocytes defective in the expression of the T-cell antigen receptor gene among radiation-exposed people. Mutat Res. 1992 Feb;265(2):173–180. doi: 10.1016/0027-5107(92)90046-5. [DOI] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H. Measurements of the frequency of human erythrocytes with gene expression loss phenotypes at the glycophorin A locus. Hum Genet. 1986 Dec;74(4):353–362. doi: 10.1007/BF00280485. [DOI] [PubMed] [Google Scholar]
- Liber H. L., Call K. M., Little J. B. Molecular and biochemical analyses of spontaneous and X-ray-induced mutants in human lymphoblastoid cells. Mutat Res. 1987 May;178(1):143–153. doi: 10.1016/0027-5107(87)90096-0. [DOI] [PubMed] [Google Scholar]
- McCarron M. A., Kutlaca A., Morley A. A. The HLA-A mutation assay: improved technique and normal results. Mutat Res. 1989 Apr;225(4):189–193. doi: 10.1016/0165-7992(89)90118-8. [DOI] [PubMed] [Google Scholar]
- McGinniss M. J., Falta M. T., Sullivan L. M., Albertini R. J. In vivo hprt mutant frequencies in T-cells of normal human newborns. Mutat Res. 1990 Feb;240(2):117–126. doi: 10.1016/0165-1218(90)90015-t. [DOI] [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Seshadri R., Ryall R. G. Measurement of in vivo mutations in human lymphocytes. Nature. 1983 Mar 10;302(5904):155–156. doi: 10.1038/302155a0. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., Falta M. T., Hunter T. C., O'Neill J. P., Jacobson-Kram D., Williams J. R., Albertini R. J. Molecular analysis of in vivo hprt mutations in human T lymphocytes. V. Effects of total body irradiation secondary to radioimmunoglobulin therapy (RIT) Mutagenesis. 1990 Sep;5(5):461–468. doi: 10.1093/mutage/5.5.461. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., O'Neill J. P., Hunter T. C., Falta M. T., Lippert M. J., Jacobson-Kram D., Williams J. R., Albertini R. J. In vivo ionizing irradiations produce deletions in the hprt gene of human T-lymphocytes. Mutat Res. 1991 Sep-Oct;250(1-2):383–396. doi: 10.1016/0027-5107(91)90195-t. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., Hunter T. C., Sullivan L. M., Nicklas J. A., Albertini R. J. Southern-blot analyses of human T-lymphocyte mutants induced in vitro by gamma-irradiation. Mutat Res. 1990 Feb;240(2):143–149. doi: 10.1016/0165-1218(90)90018-w. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., McGinniss M. J., Berman J. K., Sullivan L. M., Nicklas J. A., Albertini R. J. Refinement of a T-lymphocyte cloning assay to quantify the in vivo thioguanine-resistant mutant frequency in humans. Mutagenesis. 1987 Mar;2(2):87–94. doi: 10.1093/mutage/2.2.87. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., Sullivan L. M., Booker J. K., Pornelos B. S., Falta M. T., Greene C. J., Albertini R. J. Longitudinal study of the in vivo hprt mutant frequency in human T-lymphocytes as determined by a cell cloning assay. Environ Mol Mutagen. 1989;13(4):289–293. doi: 10.1002/em.2850130403. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Recio L., Cochrane J., Simpson D., Skopek T. R., O'Neill J. P., Nicklas J. A., Albertini R. J. DNA sequence analysis of in vivo hprt mutation in human T lymphocytes. Mutagenesis. 1990 Sep;5(5):505–510. doi: 10.1093/mutage/5.5.505. [DOI] [PubMed] [Google Scholar]
- Rossi A. M., Tates A. D., van Zeeland A. A., Vrieling H. Molecular analysis of mutations affecting hprt mRNA splicing in human T-lymphocytes in vivo. Environ Mol Mutagen. 1992;19(1):7–13. doi: 10.1002/em.2850190103. [DOI] [PubMed] [Google Scholar]
- Rossi A. M., Thijssen J. C., Tates A. D., Vrieling H., Natarajan A. T., Lohman P. H., van Zeeland A. A. Mutations affecting RNA splicing in man are detected more frequently in somatic than in germ cells. Mutat Res. 1990 Aug;244(4):353–357. doi: 10.1016/0165-7992(90)90084-w. [DOI] [PubMed] [Google Scholar]
- Simpson D., Crosby R. M., Skopek T. R. A method for specific cloning and sequencing of human hprt cDNA for mutation analysis. Biochem Biophys Res Commun. 1988 Feb 29;151(1):487–492. doi: 10.1016/0006-291x(88)90619-5. [DOI] [PubMed] [Google Scholar]
- Steingrimsdottir H., Rowley G., Dorado G., Cole J., Lehmann A. R. Mutations which alter splicing in the human hypoxanthine-guanine phosphoribosyltransferase gene. Nucleic Acids Res. 1992 Mar 25;20(6):1201–1208. doi: 10.1093/nar/20.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlé S. A., Davidson B. L., Wu V. C., Zidar F. J., Seegmiller J. E., Kelley W. N., Palella T. D. Determination of the mutations responsible for the Lesch-Nyhan syndrome in 17 subjects. Genomics. 1991 Jun;10(2):499–501. doi: 10.1016/0888-7543(91)90341-b. [DOI] [PubMed] [Google Scholar]
- Tuddenham E. G., Cooper D. N., Gitschier J., Higuchi M., Hoyer L. W., Yoshioka A., Peake I. R., Schwaab R., Olek K., Kazazian H. H. Haemophilia A: database of nucleotide substitutions, deletions, insertions and rearrangements of the factor VIII gene. Nucleic Acids Res. 1991 Sep 25;19(18):4821–4833. doi: 10.1093/nar/19.18.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling H., Thijssen J. C., Rossi A. M., van Dam F. J., Natarajan A. T., Tates A. D., van Zeeland A. A. Enhanced hprt mutant frequency but no significant difference in mutation spectrum between a smoking and a non-smoking human population. Carcinogenesis. 1992 Sep;13(9):1625–1631. doi: 10.1093/carcin/13.9.1625. [DOI] [PubMed] [Google Scholar]
- Wei S. J., Chang R. L., Wong C. Q., Bhachech N., Cui X. X., Hennig E., Yagi H., Sayer J. M., Jerina D. M., Preston B. D. Dose-dependent differences in the profile of mutations induced by an ultimate carcinogen from benzo[a]pyrene. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11227–11230. doi: 10.1073/pnas.88.24.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. L., Chen R. H., Maher V. M., McCormick J. J. Kinds and location of mutations induced by (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in the coding region of the hypoxanthine (guanine) phosphoribosyltransferase gene in diploid human fibroblasts. Carcinogenesis. 1991 Jan;12(1):71–75. doi: 10.1093/carcin/12.1.71. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Amplification and direct nucleotide sequencing of cDNA from the lysate of low numbers of diploid human cells. Gene. 1989 Nov 30;83(2):347–354. doi: 10.1016/0378-1119(89)90121-2. [DOI] [PubMed] [Google Scholar]



