Abstract
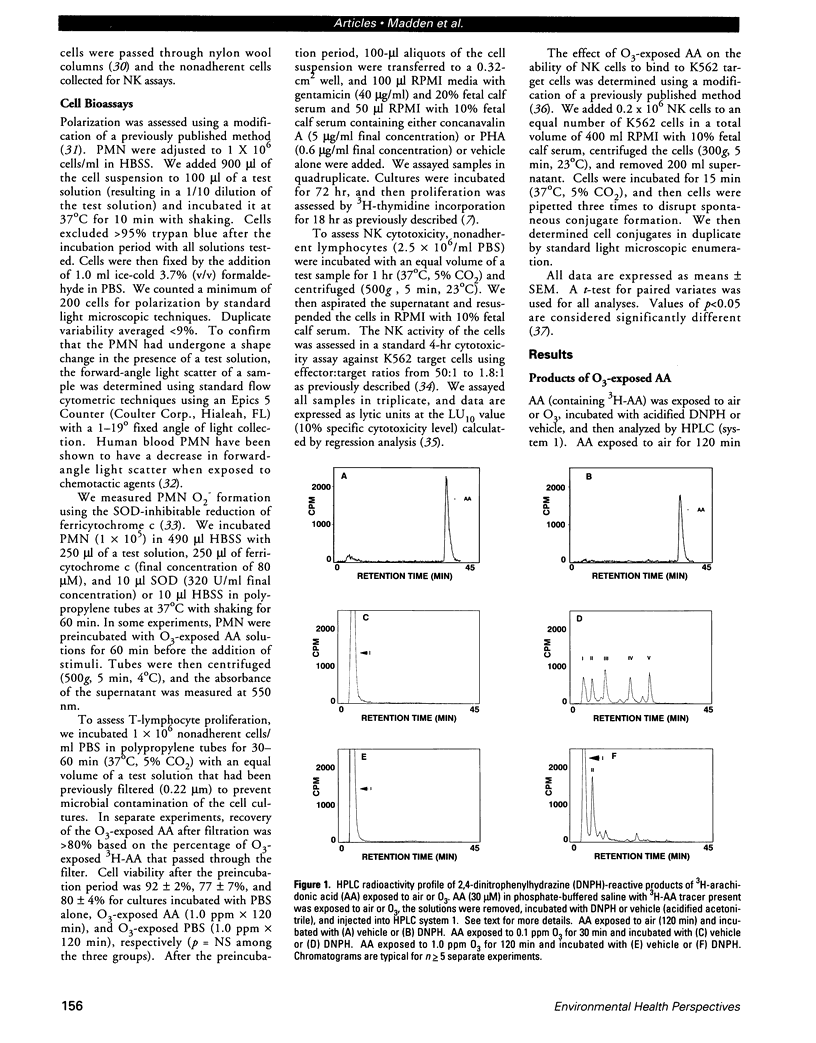
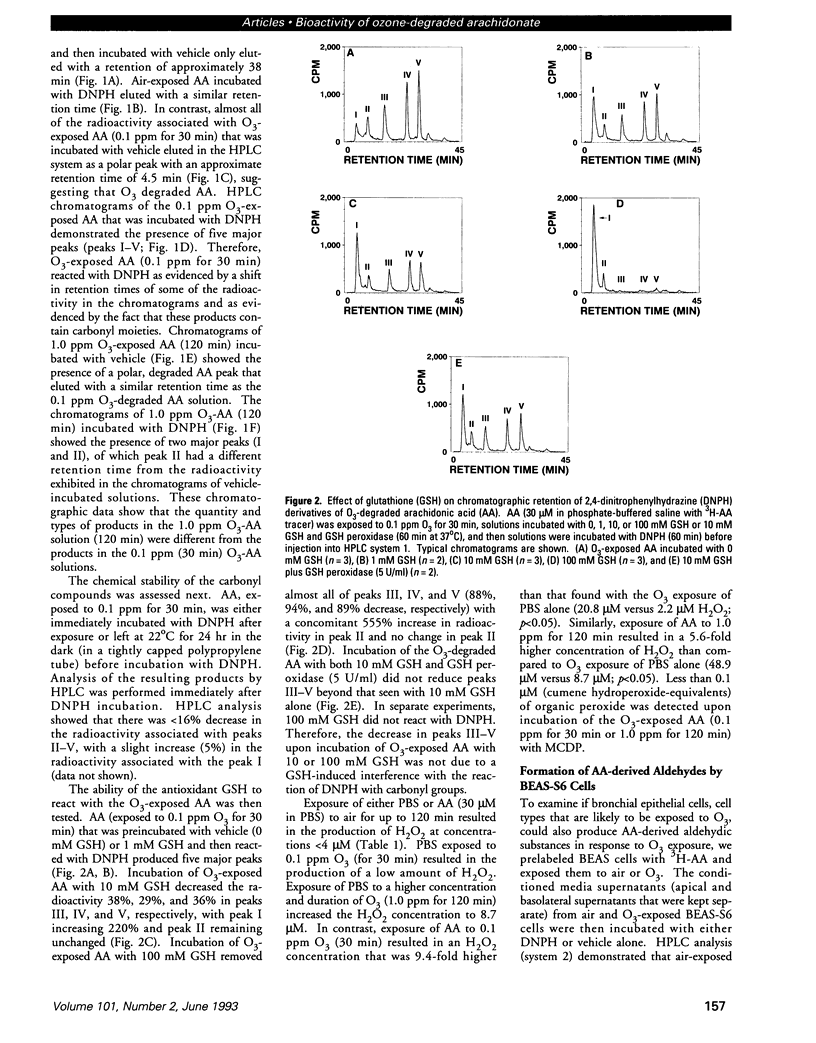
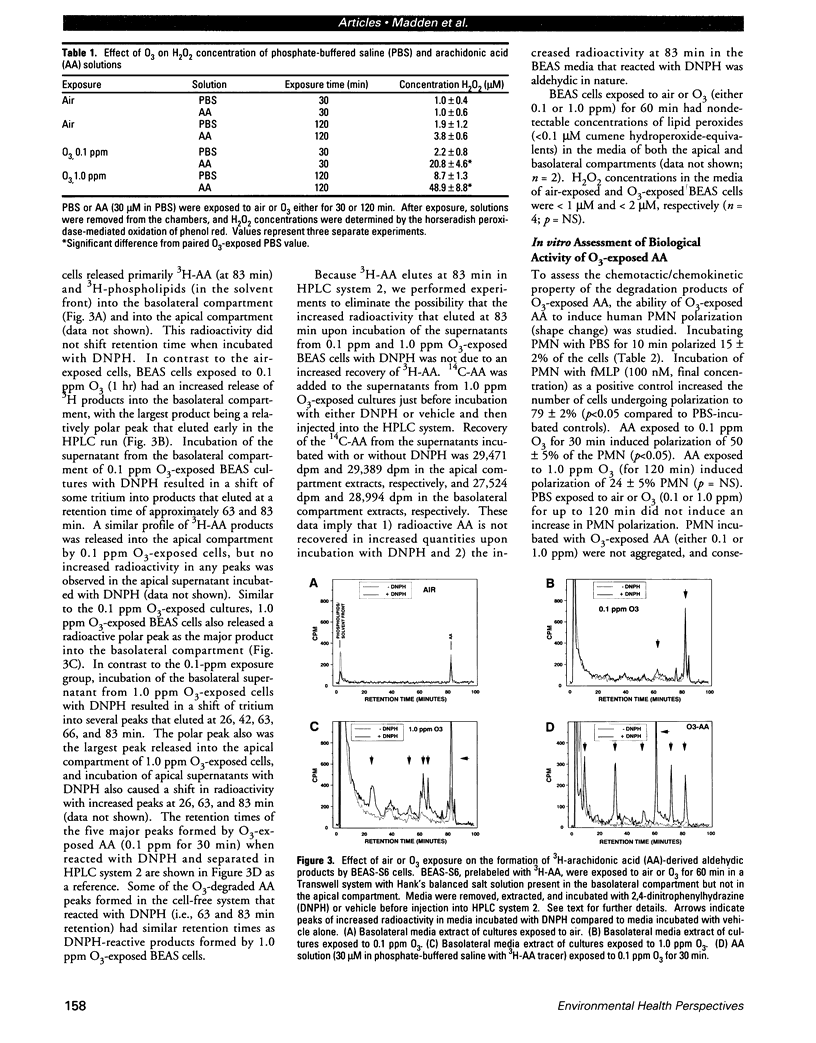
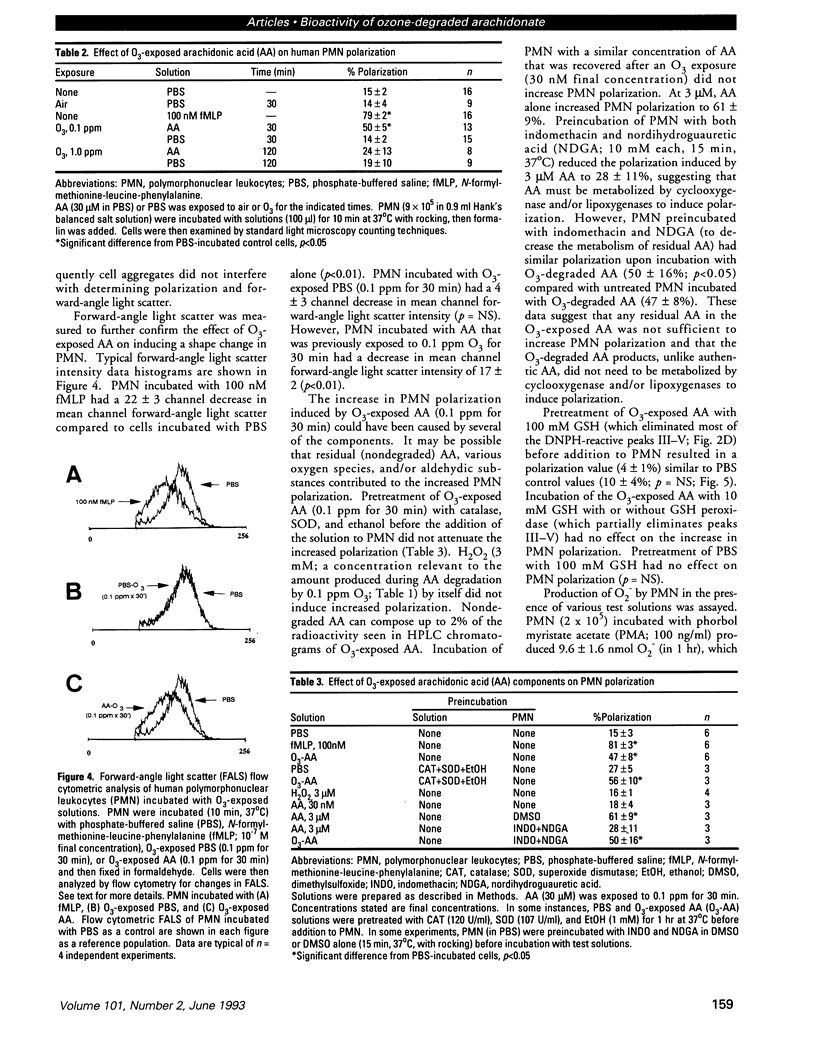
Ozone (O3) exposure in vivo has been reported to degrade arachidonic acid (AA) in the lungs of rodents. The O3-degraded AA products may play a role in the responses to this toxicant. To study the chemical nature and biological activity of O3-exposed AA, we exposed AA in a cell-free, aqueous environment to air, 0.1 ppm O3, or 1.0 ppm O3 for 30-120 min. AA exposed to air was not degraded. All O3 exposures degraded > 98% of the AA to more polar products, which were predominantly aldehydic substances (as determined by reactivity with 2,4-dinitrophenylhydrazine and subsequent separation by HPLC) and hydrogen peroxide. The type and amount of aldehydic substances formed depended on the O3 concentration and exposure duration. A human bronchial epithelial cell line (BEAS-2B, S6 subclone) exposed in vitro to either 0.1 ppm or 1.0 ppm O3 for 1 hr produced AA-derived aldehydic substances, some of which eluted with similar retention times as the aldehydic substances derived from O3 degradation of AA in the cell-free system. In vitro, O3-degraded AA induced an increase in human peripheral blood polymorphonuclear leukocyte (PMN) polarization, decreased human peripheral blood T-lymphocyte proliferation in response to mitogens, and decreased human peripheral blood natural killer cell lysis of K562 target cells. The aldehydic substances, but not hydrogen peroxide, appeared to be the principal active agents responsible for the observed effects. O3-degraded AA may play a role in the PMN influx into lungs and in decreased T-lymphocyte mitogenesis and natural killer cell activity observed in humans and rodents exposed to O3.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker S., Jordan R. L., Orlando G. S., Koren H. S. In vitro ozone exposure inhibits mitogen-induced lymphocyte proliferation and IL-2 production. J Toxicol Environ Health. 1989;26(4):469–483. doi: 10.1080/15287398909531270. [DOI] [PubMed] [Google Scholar]
- Brahmi Z., Thomas J. E., Park M., Park M., Dowdeswell I. R. The effect of acute exercise on natural killer-cell activity of trained and sedentary human subjects. J Clin Immunol. 1985 Sep;5(5):321–328. doi: 10.1007/BF00918251. [DOI] [PubMed] [Google Scholar]
- Brown G. P., Monick M. M., Hunninghake G. W. Human alveolar macrophage arachidonic acid metabolism. Am J Physiol. 1988 Jun;254(6 Pt 1):C809–C815. doi: 10.1152/ajpcell.1988.254.6.C809. [DOI] [PubMed] [Google Scholar]
- Burleson G. R., Keyes L. L., Stutzman J. D. Immunosuppression of pulmonary natural killer activity by exposure to ozone. Immunopharmacol Immunotoxicol. 1989;11(4):715–735. doi: 10.3109/08923978909005397. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., Hubbard R. C., Crystal R. G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989 Feb;139(2):370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- Curzio M., Esterbauer H., Di Mauro C., Cecchini G., Dianzani M. U. Chemotactic activity of the lipid peroxidation product 4-hydroxynonenal and homologous hydroxyalkenals. Biol Chem Hoppe Seyler. 1986 Apr;367(4):321–329. doi: 10.1515/bchm3.1986.367.1.321. [DOI] [PubMed] [Google Scholar]
- Danielson U. H., Esterbauer H., Mannervik B. Structure-activity relationships of 4-hydroxyalkenals in the conjugation catalysed by mammalian glutathione transferases. Biochem J. 1987 Nov 1;247(3):707–713. doi: 10.1042/bj2470707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. B., McDonnell W. F., Mann R., Becker S., House D. E., Schreinemachers D., Koren H. S. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol. 1991 Jan;4(1):72–81. doi: 10.1165/ajrcmb/4.1.72. [DOI] [PubMed] [Google Scholar]
- Donabedian H., Sawyer T., Senitzer D. Inhibition of neutrophil shape change by an inhibitor of chemotaxis. J Leukoc Biol. 1987 Nov;42(5):510–518. doi: 10.1002/jlb.42.5.510. [DOI] [PubMed] [Google Scholar]
- Dungworth D. L., Castleman W. L., Chow C. K., Mellick P. W., Mustafa M. G., Tarkington B., Tyler W. S. Effect of ambient levels of ozone on monkeys. Fed Proc. 1975 Jul;34(8):1670–1674. [PubMed] [Google Scholar]
- El-Hag A., Clark R. A. Down-regulation of human natural killer activity against tumors by the neutrophil myeloperoxidase system and hydrogen peroxide. J Immunol. 1984 Dec;133(6):3291–3297. [PubMed] [Google Scholar]
- Friedman M., Madden M. C., Samet J. M., Koren H. S. Effects of ozone exposure on lipid metabolism in human alveolar macrophages. Environ Health Perspect. 1992 Jul;97:95–101. doi: 10.1289/ehp.929795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. T., Cianciolo G. J., Snyderman R., Argov S., Koren H. S. Inhibition of human natural killer cell activity by a synthetic peptide homologous to a conserved region in the retroviral protein, p15E. J Immunol. 1987 Feb 1;138(3):889–894. [PubMed] [Google Scholar]
- Henke D., Danilowicz R., Eling T. Arachidonic acid metabolism by isolated epidermal basal and differentiated keratinocytes from the hairless mouse. Biochim Biophys Acta. 1986 Apr 15;876(2):271–279. doi: 10.1016/0005-2760(86)90284-5. [DOI] [PubMed] [Google Scholar]
- Ke Y., Reddel R. R., Gerwin B. I., Miyashita M., McMenamin M., Lechner J. F., Harris C. C. Human bronchial epithelial cells with integrated SV40 virus T antigen genes retain the ability to undergo squamous differentiation. Differentiation. 1988 Jun;38(1):60–66. doi: 10.1111/j.1432-0436.1988.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Brandt C. P., Tso C. Y., Laszlo J. Modulation of natural killing activity by lymphoblastoid interferon in cancer patients. J Biol Response Mod. 1983;2(2):151–165. [PubMed] [Google Scholar]
- Koren H. S., Devlin R. B., Graham D. E., Mann R., McGee M. P., Horstman D. H., Kozumbo W. J., Becker S., House D. E., McDonnell W. F. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis. 1989 Feb;139(2):407–415. doi: 10.1164/ajrccm/139.2.407. [DOI] [PubMed] [Google Scholar]
- Leikauf G. D., Driscoll K. E., Wey H. E. Ozone-induced augmentation of eicosanoid metabolism in epithelial cells from bovine trachea. Am Rev Respir Dis. 1988 Feb;137(2):435–442. doi: 10.1164/ajrccm/137.2.435. [DOI] [PubMed] [Google Scholar]
- Madden M. C., Eling T. E., Dailey L. A., Friedman M. The effect of ozone exposure on rat alveolar macrophage arachidonic acid metabolism. Exp Lung Res. 1991 Jan-Feb;17(1):47–63. doi: 10.3109/01902149109063281. [DOI] [PubMed] [Google Scholar]
- McKinnon K. P., Madden M. C., Noah T. L., Devlin R. B. In vitro ozone exposure increases release of arachidonic acid products from a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1993 Feb;118(2):215–223. doi: 10.1006/taap.1993.1027. [DOI] [PubMed] [Google Scholar]
- Mellick P. W., Dungworth D. L., Schwartz L. W., Tyler W. S. Short term morphologic effects of high ambient levels of ozone on lungs of rhesus monkeys. Lab Invest. 1977 Jan;36(1):82–90. [PubMed] [Google Scholar]
- Mercado C., Molina F., Navas J., Quiñones C., Eylar E. H. Inhibition of T cell mitogenesis by nitrofurans. Biochem Pharmacol. 1991 Feb 15;41(4):503–508. doi: 10.1016/0006-2952(91)90621-b. [DOI] [PubMed] [Google Scholar]
- Noah T. L., Paradiso A. M., Madden M. C., McKinnon K. P., Devlin R. B. The response of a human bronchial epithelial cell line to histamine: intracellular calcium changes and extracellular release of inflammatory mediators. Am J Respir Cell Mol Biol. 1991 Nov;5(5):484–492. doi: 10.1165/ajrcmb/5.5.484. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts? Free Radic Biol Med. 1992;12(1):83–88. doi: 10.1016/0891-5849(92)90060-t. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Miki M., Das B., Church D. F. The mixture of aldehydes and hydrogen peroxide produced in the ozonation of dioleoyl phosphatidylcholine causes hemolysis of human red blood cells. Chem Biol Interact. 1991;79(1):41–52. doi: 10.1016/0009-2797(91)90051-8. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J. L., Bassett D. J. Effect of 2 ppm ozone exposure on rat lung lipid fatty acids. Exp Lung Res. 1988;14(4):477–489. doi: 10.3109/01902148809087822. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Ke Y., Gerwin B. I., McMenamin M. G., Lechner J. F., Su R. T., Brash D. E., Park J. B., Rhim J. S., Harris C. C. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988 Apr 1;48(7):1904–1909. [PubMed] [Google Scholar]
- Rossi M. A., Curzio M., Di Mauro C., Fidale F., Garramone A., Esterbauer H., Torrielli M., Dianzani M. U. Experimental studies on the mechanism of action of 4-hydroxy-2,3-trans-nonenal, a lipid peroxidation product displaying chemotactic activity toward rat neutrophils. Cell Biochem Funct. 1991 Jul;9(3):163–170. doi: 10.1002/cbf.290090304. [DOI] [PubMed] [Google Scholar]
- Sadana T., Dhall K., Sanyal S. N., Wali A., Minocha R., Majumdar S. Isolation and chemical composition of surface-active material from human lung lavage. Lipids. 1988 Jun;23(6):551–558. doi: 10.1007/BF02535596. [DOI] [PubMed] [Google Scholar]
- Samet J. M., Noah T. L., Devlin R. B., Yankaskas J. R., McKinnon K., Dailey L. A., Friedman M. Effect of ozone on platelet-activating factor production in phorbol-differentiated HL60 cells, a human bronchial epithelial cell line (BEAS S6), and primary human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1992 Nov;7(5):514–522. doi: 10.1165/ajrcmb/7.5.514. [DOI] [PubMed] [Google Scholar]
- Santrock J., Gorski R. A., O'Gara J. F. Products and mechanism of the reaction of ozone with phospholipids in unilamellar phospholipid vesicles. Chem Res Toxicol. 1992 Jan-Feb;5(1):134–141. doi: 10.1021/tx00025a023. [DOI] [PubMed] [Google Scholar]
- Seltzer J., Bigby B. G., Stulbarg M., Holtzman M. J., Nadel J. A., Ueki I. F., Leikauf G. D., Goetzl E. J., Boushey H. A. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol (1985) 1986 Apr;60(4):1321–1326. doi: 10.1152/jappl.1986.60.4.1321. [DOI] [PubMed] [Google Scholar]
- Shimasaki H., Takatori T., Anderson W. R., Horten H. L., Privett O. S. Alteration of lung lipids in ozone exposed rats. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1256–1262. doi: 10.1016/0006-291x(76)90332-6. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn P. H., Murphy T. M., Peters-Golden M. Glucocorticoids fail to inhibit arachidonic acid metabolism stimulated by hydrogen peroxide in the alveolar macrophage. J Leukoc Biol. 1990 Jul;48(1):81–88. doi: 10.1002/jlb.48.1.81. [DOI] [PubMed] [Google Scholar]
- Stremler K. E., Stafforini D. M., Prescott S. M., Zimmerman G. A., McIntyre T. M. An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. J Biol Chem. 1989 Apr 5;264(10):5331–5334. [PubMed] [Google Scholar]
- Van Loveren H., Krajnc E. I., Rombout P. J., Blommaert F. A., Vos J. G. Effects of ozone, hexachlorobenzene, and bis(tri-n-butyltin)oxide on natural killer activity in the rat lung. Toxicol Appl Pharmacol. 1990 Jan;102(1):21–33. doi: 10.1016/0041-008x(90)90080-e. [DOI] [PubMed] [Google Scholar]
- Van Scott M. R., McIntire M. R., Henke D. C. Arachidonic acid metabolism and regulation of ion transport in rabbit Clara cells. Am J Physiol. 1990 Oct;259(4 Pt 1):L213–L221. doi: 10.1152/ajplung.1990.259.4.L213. [DOI] [PubMed] [Google Scholar]
- Witz G., Lawrie N. J., Amoruso M. A., Goldstein B. D. Inhibition by reactive aldehydes of superoxide anion radical production from stimulated polymorphonuclear leukocytes and pulmonary alveolar macrophages. Effects on cellular sulfhydryl groups and NADPH oxidase activity. Biochem Pharmacol. 1987 Mar 1;36(5):721–726. doi: 10.1016/0006-2952(87)90725-8. [DOI] [PubMed] [Google Scholar]
- Zoschke D. C., Staite N. D. Suppression of human lymphocyte proliferation by activated neutrophils or H2O2: surviving cells have an altered T helper/T suppressor ratio and an increased resistance to secondary oxidant exposure. Clin Immunol Immunopathol. 1987 Feb;42(2):160–170. doi: 10.1016/0090-1229(87)90003-1. [DOI] [PubMed] [Google Scholar]


