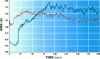
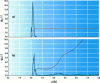
Abstract
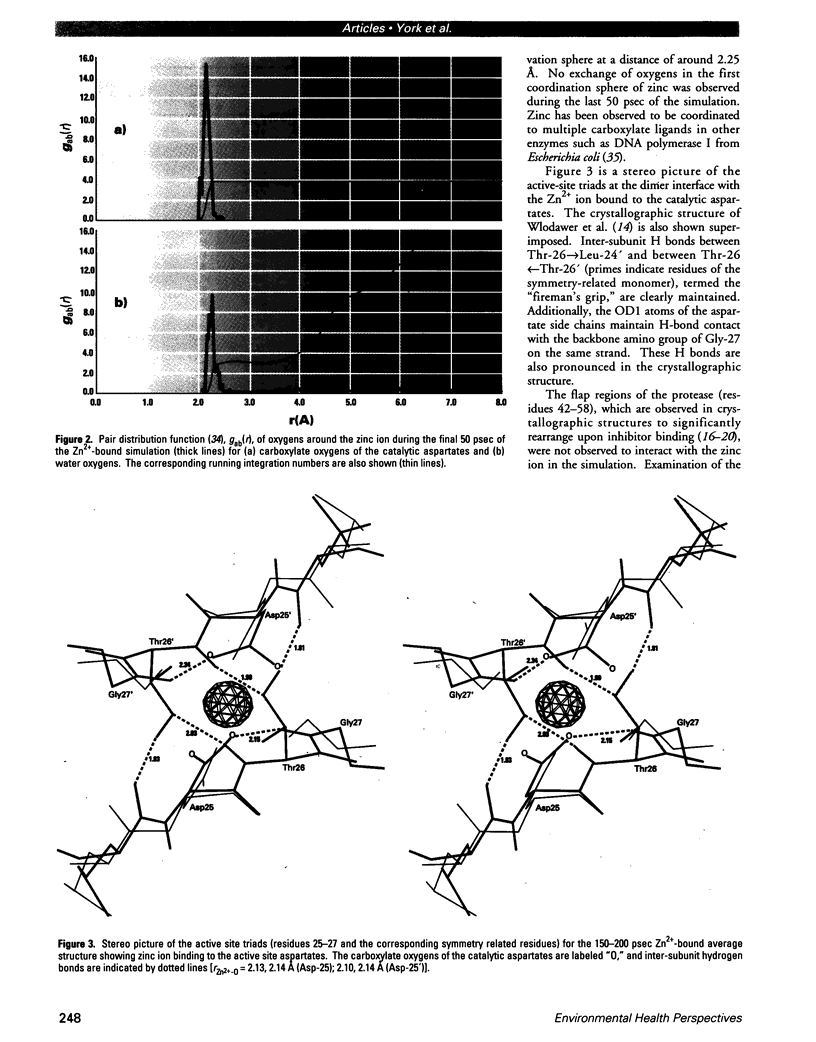
Human immunodeficiency virus type 1 protease is inhibited in vitro by zinc ions at neutral pH. The binding site of these ions is not known; however, experimental data suggest that binding may occur in the active site. To examine the possibility of zinc binding in the active site, molecular dynamics simulations in the presence and absence of zinc have been carried out to 200 psec. The results are compared with the 2.8-A crystallographic structures of a synthetic HIV-1 protease, and a zinc binding site at the catalytic aspartate residues (Asp-25, Asp-25') is proposed. Molecular dynamics simulations show that the zinc ion remains stably bound in this region, coordinating the carboxylate side chains of both aspartate residues. Interaction with zinc does not disrupt the dimeric structure of the protein or significantly alter the structure of the active site. These data are consistent with experimental studies of HIV-1 protease inhibition by zinc and give strong evidence that this is the binding site that leads to inactivation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beese L. S., Steitz T. A. Structural basis for the 3'-5' exonuclease activity of Escherichia coli DNA polymerase I: a two metal ion mechanism. EMBO J. 1991 Jan;10(1):25–33. doi: 10.1002/j.1460-2075.1991.tb07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke P. L., Leu C. T., Davis L. J., Heimbach J. C., Diehl R. E., Hill W. S., Dixon R. A., Sigal I. S. Human immunodeficiency virus protease. Bacterial expression and characterization of the purified aspartic protease. J Biol Chem. 1989 Feb 5;264(4):2307–2312. [PubMed] [Google Scholar]
- Debouck C., Gorniak J. G., Strickler J. E., Meek T. D., Metcalf B. W., Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck C. The HIV-1 protease as a therapeutic target for AIDS. AIDS Res Hum Retroviruses. 1992 Feb;8(2):153–164. doi: 10.1089/aid.1992.8.153. [DOI] [PubMed] [Google Scholar]
- Erickson J., Neidhart D. J., VanDrie J., Kempf D. J., Wang X. C., Norbeck D. W., Plattner J. J., Rittenhouse J. W., Turon M., Wideburg N. Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990 Aug 3;249(4968):527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. M., McKeever B. M., VanMiddlesworth J. F., Springer J. P., Heimbach J. C., Leu C. T., Herber W. K., Dixon R. A., Darke P. L. Crystallographic analysis of a complex between human immunodeficiency virus type 1 protease and acetyl-pepstatin at 2.0-A resolution. J Biol Chem. 1990 Aug 25;265(24):14209–14219. [PubMed] [Google Scholar]
- Foley C. K., Pedersen L. G., Charifson P. S., Darden T. A., Wittinghofer A., Pai E. F., Anderson M. W. Simulation of the solution structure of the H-ras p21-GTP complex. Biochemistry. 1992 Jun 2;31(21):4951–4959. doi: 10.1021/bi00136a005. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Meidel M. C., Pan Y. C., Manneberg M., Lahm H. W., Grüninger-Leitch F. Identification of a human immunodeficiency virus-1 protease cleavage site within the 66,000 Dalton subunit of reverse transcriptase. Biochem Biophys Res Commun. 1990 Apr 16;168(1):30–36. doi: 10.1016/0006-291x(90)91670-n. [DOI] [PubMed] [Google Scholar]
- Gustchina A., Weber I. T. Comparative analysis of the sequences and structures of HIV-1 and HIV-2 proteases. Proteins. 1991;10(4):325–339. doi: 10.1002/prot.340100406. [DOI] [PubMed] [Google Scholar]
- Huff J. R. HIV protease: a novel chemotherapeutic target for AIDS. J Med Chem. 1991 Aug;34(8):2305–2314. doi: 10.1021/jm00112a001. [DOI] [PubMed] [Google Scholar]
- Hyland L. J., Tomaszek T. A., Jr, Meek T. D. Human immunodeficiency virus-1 protease. 2. Use of pH rate studies and solvent kinetic isotope effects to elucidate details of chemical mechanism. Biochemistry. 1991 Aug 27;30(34):8454–8463. doi: 10.1021/bi00098a024. [DOI] [PubMed] [Google Scholar]
- Ido E., Han H. P., Kezdy F. J., Tang J. Kinetic studies of human immunodeficiency virus type 1 protease and its active-site hydrogen bond mutant A28S. J Biol Chem. 1991 Dec 25;266(36):24359–24366. [PubMed] [Google Scholar]
- Jaskólski M., Tomasselli A. G., Sawyer T. K., Staples D. G., Heinrikson R. L., Schneider J., Kent S. B., Wlodawer A. Structure at 2.5-A resolution of chemically synthesized human immunodeficiency virus type 1 protease complexed with a hydroxyethylene-based inhibitor. Biochemistry. 1991 Feb 12;30(6):1600–1609. doi: 10.1021/bi00220a023. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R., Blundell T., Hemmings A., Overington J., Wilderspin A., Wood S., Merson J. R., Whittle P. J., Danley D. E., Geoghegan K. F. X-ray analysis of HIV-1 proteinase at 2.7 A resolution confirms structural homology among retroviral enzymes. Nature. 1989 Nov 16;342(6247):299–302. doi: 10.1038/342299a0. [DOI] [PubMed] [Google Scholar]
- Lybrand T. P., McCammon J. A., Wipff G. Theoretical calculation of relative binding affinity in host-guest systems. Proc Natl Acad Sci U S A. 1986 Feb;83(4):833–835. doi: 10.1073/pnas.83.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek T. D., Dayton B. D., Metcalf B. W., Dreyer G. B., Strickler J. E., Gorniak J. G., Rosenberg M., Moore M. L., Magaard V. W., Debouck C. Human immunodeficiency virus 1 protease expressed in Escherichia coli behaves as a dimeric aspartic protease. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1841–1845. doi: 10.1073/pnas.86.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Schneider J., Sathyanarayana B. K., Toth M. V., Marshall G. R., Clawson L., Selk L., Kent S. B., Wlodawer A. Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 A resolution. Science. 1989 Dec 1;246(4934):1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- Richards A. D., Roberts R., Dunn B. M., Graves M. C., Kay J. Effective blocking of HIV-1 proteinase activity by characteristic inhibitors of aspartic proteinases. FEBS Lett. 1989 Apr 10;247(1):113–117. doi: 10.1016/0014-5793(89)81251-7. [DOI] [PubMed] [Google Scholar]
- Seelmeier S., Schmidt H., Turk V., von der Helm K. Human immunodeficiency virus has an aspartic-type protease that can be inhibited by pepstatin A. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6612–6616. doi: 10.1073/pnas.85.18.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain A. L., Miller M. M., Green J., Rich D. H., Schneider J., Kent S. B., Wlodawer A. X-ray crystallographic structure of a complex between a synthetic protease of human immunodeficiency virus 1 and a substrate-based hydroxyethylamine inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8805–8809. doi: 10.1073/pnas.87.22.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Kikuno R., Hayashida H., Miyata T., Kugimiya W., Inouye S., Yuki S., Saigo K. Close structural resemblance between putative polymerase of a Drosophila transposable genetic element 17.6 and pol gene product of Moloney murine leukaemia virus. EMBO J. 1985 May;4(5):1267–1272. doi: 10.1002/j.1460-2075.1985.tb03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasselli A. G., Hui J. O., Sawyer T. K., Staples D. J., Bannow C., Reardon I. M., Howe W. J., DeCamp D. L., Craik C. S., Heinrikson R. L. Specificity and inhibition of proteases from human immunodeficiency viruses 1 and 2. J Biol Chem. 1990 Aug 25;265(24):14675–14683. [PubMed] [Google Scholar]
- Weber I. T. Comparison of the crystal structures and intersubunit interactions of human immunodeficiency and Rous sarcoma virus proteases. J Biol Chem. 1990 Jun 25;265(18):10492–10496. [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]
- Woon T. C., Brinkworth R. I., Fairlie D. P. Inhibition of HIV-1 proteinase by metal ions. Int J Biochem. 1992 Jun;24(6):911–914. doi: 10.1016/0020-711x(92)90096-j. [DOI] [PubMed] [Google Scholar]
- York D. M., Darden T. A., Pedersen L. G., Anderson M. W. Molecular dynamics simulation of HIV-1 protease in a crystalline environment and in solution. Biochemistry. 1993 Feb 16;32(6):1443–1453. doi: 10.1021/bi00057a007. [DOI] [PubMed] [Google Scholar]