Abstract
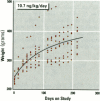
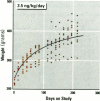
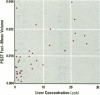
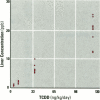
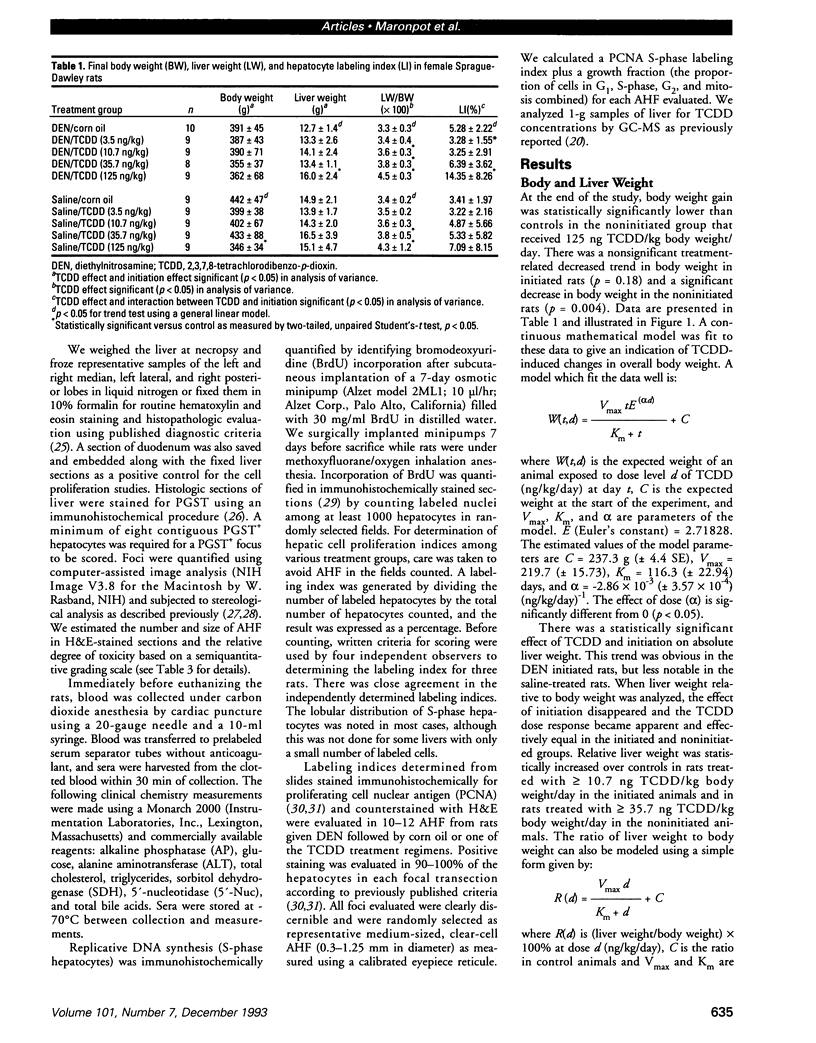
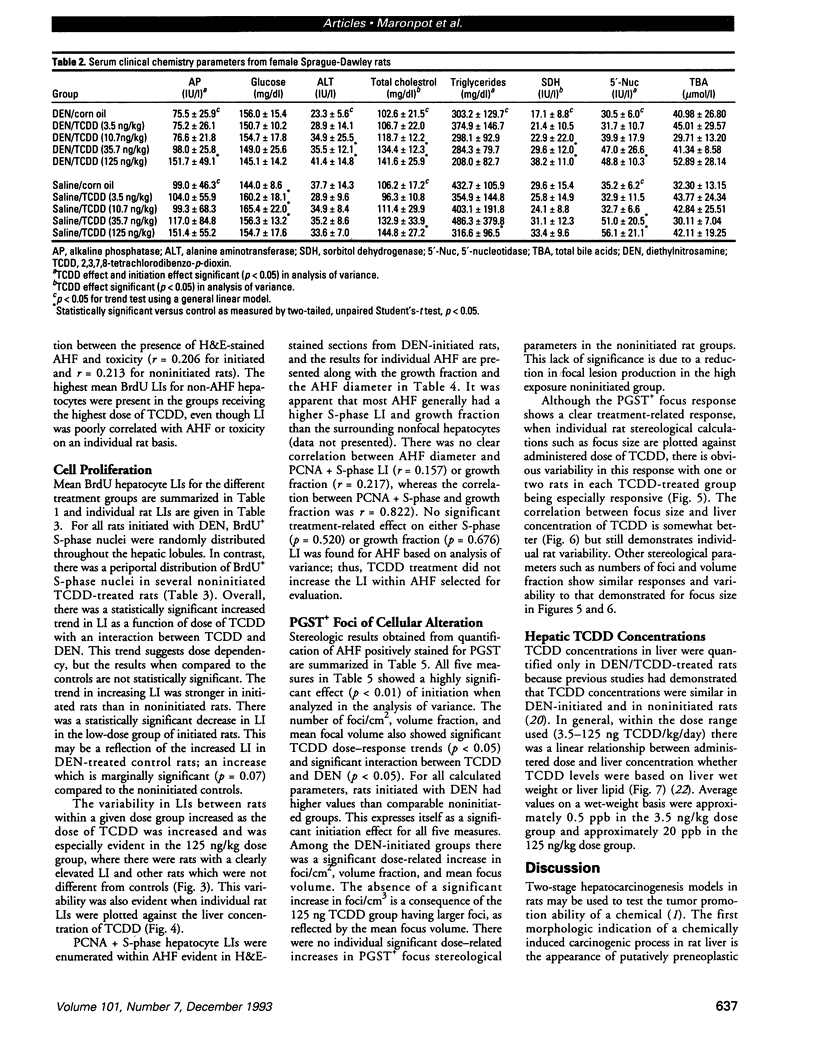
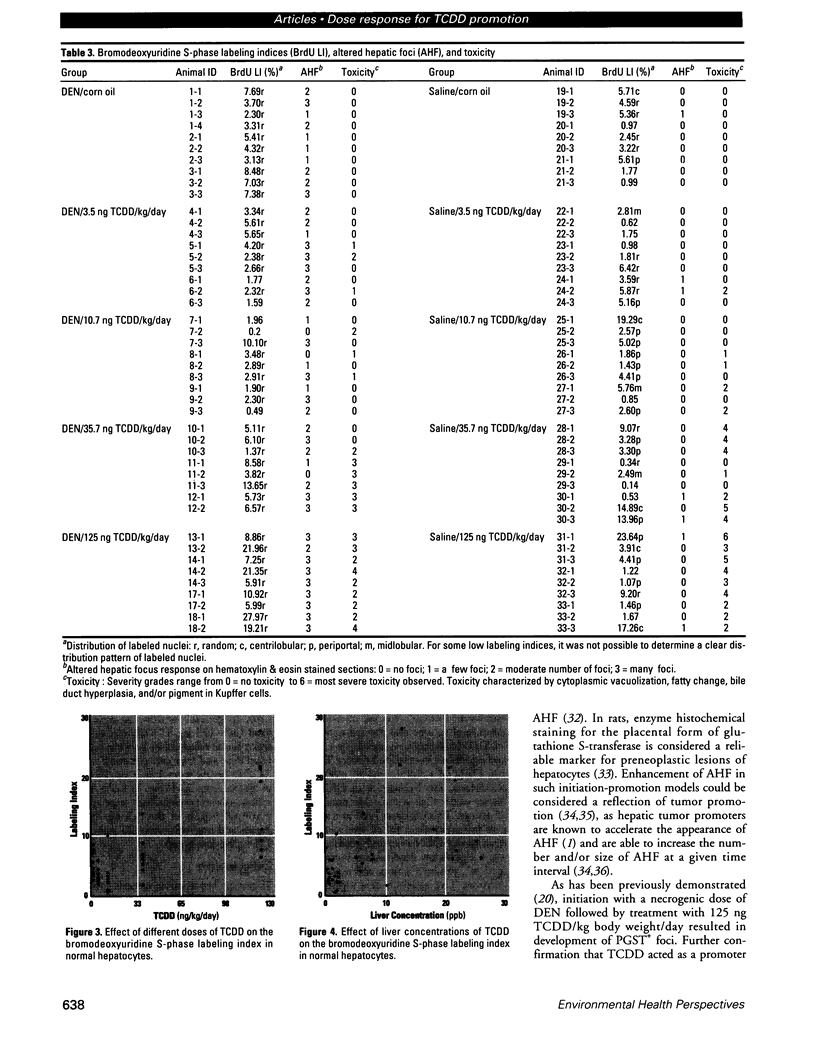
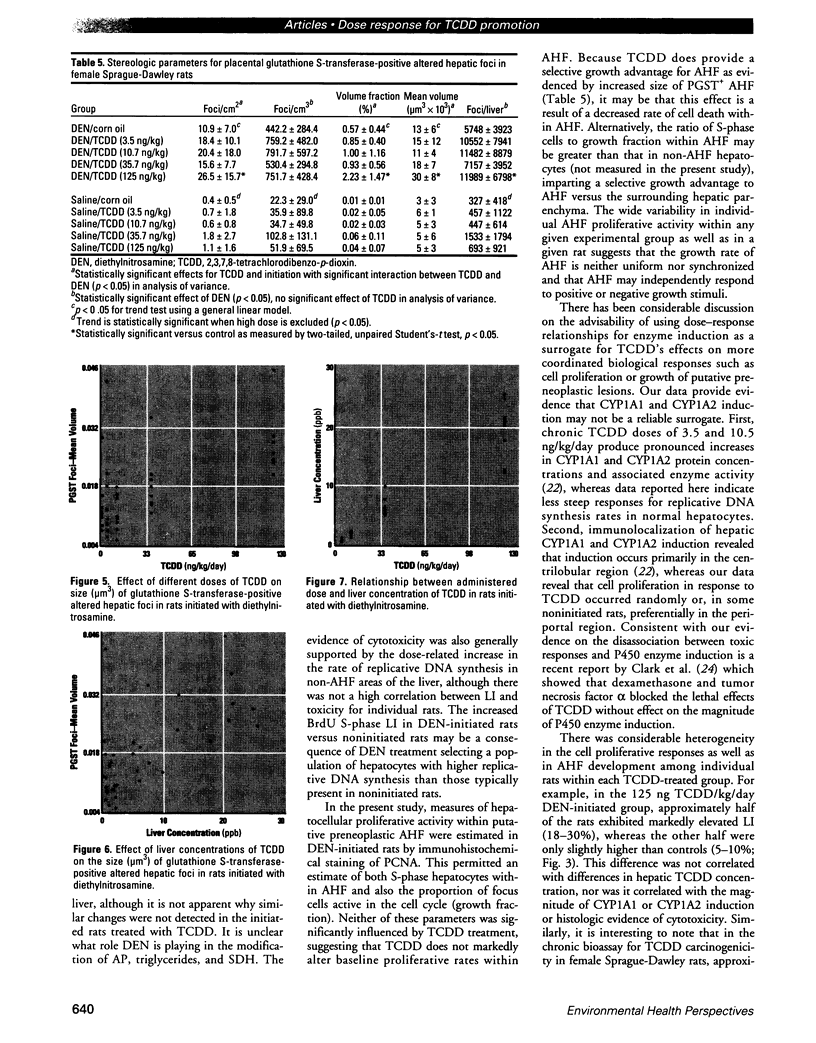
The present study examines the dose-response relationship for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) promotion of histologic and biochemical parameters by using a two-stage model for hepatocarcinogenesis in female Sprague-Dawley rats initiated with a single intraperitoneal dose of 175 mg of diethylnitrosamine (DEN)/kg body weight at 70 days of age. Starting 2 weeks after initiation, treatment groups of 8-10 rats were given TCDD by gavage in corn oil once every 2 weeks for 30 weeks. Doses were 3.5, 10.7, 35.7, and 125 ng TCDD/kg body weight/day. A significant body weight reduction was present in the noninitiated group that received 125 ng TCDD. Relative liver weight was statistically increased in initiated rats treated with > or = 10.7 ng TCDD and in noninitiated rats treated with > or = 35.7 ng TCDD. Histopathologic evidence of cytotoxicity was dose-related in all TCDD-treated groups. There was a statistically significant dose response in the bromodeoxyuridine (BrdU) S-phase labeling index (LI) in the DEN-initiated rats (p < 0.01) and a marginally significant trend in the saline-treated rats (p = 0.10), but proliferating cell nuclear antigen S-phase LI and growth fraction within altered hepatic foci showed no increase. Among the DEN-initiated groups there was a significant increase in glutathione S-transferase altered hepatic foci stereological parameters in the 125 ng TCDD group. This study demonstrates that dose-response relationships for TCDD's effects on cell proliferation growth of altered hepatic foci are different from previously reported effects on P450 gene expression, indicating that different biological or biochemical responses may exhibit different dose-response relationships.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham K., Krowke R., Neubert D. Pharmacokinetics and biological activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. 1. Dose-dependent tissue distribution and induction of hepatic ethoxyresorufin O-deethylase in rats following a single injection. Arch Toxicol. 1988;62(5):359–368. doi: 10.1007/BF00293624. [DOI] [PubMed] [Google Scholar]
- Campbell H. A., Pitot H. C., Potter V. R., Laishes B. A. Application of quantitative stereology to the evaluation of enzyme-altered foci in rat liver. Cancer Res. 1982 Feb;42(2):465–472. [PubMed] [Google Scholar]
- Cohen S. M., Ellwein L. B. Cell proliferation in carcinogenesis. Science. 1990 Aug 31;249(4972):1007–1011. doi: 10.1126/science.2204108. [DOI] [PubMed] [Google Scholar]
- Dragan Y. P., Rizvi T., Xu Y. H., Hully J. R., Bawa N., Campbell H. A., Maronpot R. R., Pitot H. C. An initiation-promotion assay in rat liver as a potential complement to the 2-year carcinogenesis bioassay. Fundam Appl Toxicol. 1991 Apr;16(3):525–547. doi: 10.1016/0272-0590(91)90093-j. [DOI] [PubMed] [Google Scholar]
- Foley J. F., Tuck P. D., Ton T. V., Frost M., Kari F., Anderson M. W., Maronpot R. R. Inhalation exposure to a hepatocarcinogenic concentration of methylene chloride does not induce sustained replicative DNA synthesis in hepatocytes of female B6C3F1 mice. Carcinogenesis. 1993 May;14(5):811–817. doi: 10.1093/carcin/14.5.811. [DOI] [PubMed] [Google Scholar]
- Goldsworthy T. L., Hanigan M. H., Pitot H. C. Models of hepatocarcinogenesis in the rat--contrasts and comparisons. Crit Rev Toxicol. 1986;17(1):61–89. doi: 10.3109/10408448609037071. [DOI] [PubMed] [Google Scholar]
- Goldsworthy T. L., Pitot H. C. The quantitative analysis and stability of histochemical markers of altered hepatic foci in rat liver following initiation by diethylnitrosamine administration and promotion with phenobarbital. Carcinogenesis. 1985 Sep;6(9):1261–1269. doi: 10.1093/carcin/6.9.1261. [DOI] [PubMed] [Google Scholar]
- Graham M. J., Lucier G. W., Linko P., Maronpot R. R., Goldstein J. A. Increases in cytochrome P-450 mediated 17 beta-estradiol 2-hydroxylase activity in rat liver microsomes after both acute administration and subchronic administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin in a two-stage hepatocarcinogenesis model. Carcinogenesis. 1988 Nov;9(11):1935–1941. doi: 10.1093/carcin/9.11.1935. [DOI] [PubMed] [Google Scholar]
- Hudson L. G., Toscano W. A., Jr, Greenlee W. F. Regulation of epidermal growth factor binding in a human keratinocyte cell line by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1985 Feb;77(2):251–259. doi: 10.1016/0041-008x(85)90324-2. [DOI] [PubMed] [Google Scholar]
- Huff J. E., Salmon A. G., Hooper N. K., Zeise L. Long-term carcinogenesis studies on 2,3,7,8-tetrachlorodibenzo-p-dioxin and hexachlorodibenzo-p-dioxins. Cell Biol Toxicol. 1991 Jan;7(1):67–94. doi: 10.1007/BF00121331. [DOI] [PubMed] [Google Scholar]
- Ito N., Tatematsu M., Hasegawa R., Tsuda H. Medium-term bioassay system for detection of carcinogens and modifiers of hepatocarcinogenesis utilizing the GST-P positive liver cell focus as an endpoint marker. Toxicol Pathol. 1989;17(4 Pt 1):630–641. doi: 10.1177/0192623389017004108. [DOI] [PubMed] [Google Scholar]
- Kociba R. J., Keyes D. G., Beyer J. E., Carreon R. M., Wade C. E., Dittenber D. A., Kalnins R. P., Frauson L. E., Park C. N., Barnard S. D. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol. 1978 Nov;46(2):279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- Kohn M. C., Lucier G. W., Clark G. C., Sewall C., Tritscher A. M., Portier C. J. A mechanistic model of effects of dioxin on gene expression in the rat liver. Toxicol Appl Pharmacol. 1993 May;120(1):138–154. doi: 10.1006/taap.1993.1096. [DOI] [PubMed] [Google Scholar]
- Leonard T. B., Adams T., Popp J. A. Dinitrotoluene isomer-specific enhancement of the expression of diethylnitrosamine-initiated hepatocyte foci. Carcinogenesis. 1986 Nov;7(11):1797–1803. doi: 10.1093/carcin/7.11.1797. [DOI] [PubMed] [Google Scholar]
- Lucier G. W., Rumbaugh R. C., McCoy Z., Hass R., Harvan D., Albro P. Ingestion of soil contaminated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters hepatic enzyme activities in rats. Fundam Appl Toxicol. 1986 Feb;6(2):364–371. doi: 10.1016/0272-0590(86)90252-6. [DOI] [PubMed] [Google Scholar]
- Lucier G. W., Tritscher A., Goldsworthy T., Foley J., Clark G., Goldstein J., Maronpot R. Ovarian hormones enhance 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated increases in cell proliferation and preneoplastic foci in a two-stage model for rat hepatocarcinogenesis. Cancer Res. 1991 Mar 1;51(5):1391–1397. [PubMed] [Google Scholar]
- Madhukar B. V., Brewster D. W., Matsumura F. Effects of in vivo-administered 2,3,7,8-tetrachlorodibenzo-p-dioxin on receptor binding of epidermal growth factor in the hepatic plasma membrane of rat, guinea pig, mouse, and hamster. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7407–7411. doi: 10.1073/pnas.81.23.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A., Onodera H., Ogasawara H., Matsushima Y., Mitsumori K., Hayashi Y. Threshold dose dependence in phenobarbital promotion of rat hepatocarcinogenesis initiated by diethylnitrosamine. Carcinogenesis. 1992 Mar;13(3):501–503. doi: 10.1093/carcin/13.3.501. [DOI] [PubMed] [Google Scholar]
- Maronpot R. R., Montgomery C. A., Jr, Boorman G. A., McConnell E. E. National Toxicology Program nomenclature for hepatoproliferative lesions of rats. Toxicol Pathol. 1986;14(2):263–273. doi: 10.1177/019262338601400217. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Goldsworthy T. L., Moran S., Kennan W., Glauert H. P., Maronpot R. R., Campbell H. A. A method to quantitate the relative initiating and promoting potencies of hepatocarcinogenic agents in their dose-response relationships to altered hepatic foci. Carcinogenesis. 1987 Oct;8(10):1491–1499. doi: 10.1093/carcin/8.10.1491. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Goldsworthy T., Campbell H. A., Poland A. Quantitative evaluation of the promotion by 2,3,7,8-tetrachlorodibenzo-p-dioxin of hepatocarcinogenesis from diethylnitrosamine. Cancer Res. 1980 Oct;40(10):3616–3620. [PubMed] [Google Scholar]
- Poland A., Glover E. An estimate of the maximum in vivo covalent binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to rat liver protein, ribosomal RNA, and DNA. Cancer Res. 1979 Sep;39(9):3341–3344. [PubMed] [Google Scholar]
- Poland A., Palen D., Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982 Nov 18;300(5889):271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Portier C., Tritscher A., Kohn M., Sewall C., Clark G., Edler L., Hoel D., Lucier G. Ligand/receptor binding for 2,3,7,8-TCDD: implications for risk assessment. Fundam Appl Toxicol. 1993 Jan;20(1):48–56. doi: 10.1006/faat.1993.1006. [DOI] [PubMed] [Google Scholar]
- Pugh T. D., King J. H., Koen H., Nychka D., Chover J., Wahba G., He Y. H., Goldfarb S. Reliable stereological method for estimating the number of microscopic hepatocellular foci from their transections. Cancer Res. 1983 Mar;43(3):1261–1268. [PubMed] [Google Scholar]
- Romkes M., Piskorska-Pliszczynska J., Safe S. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic and uterine estrogen receptor levels in rats. Toxicol Appl Pharmacol. 1987 Feb;87(2):306–314. doi: 10.1016/0041-008x(87)90292-4. [DOI] [PubMed] [Google Scholar]
- Sato K. Glutathione S-transferases and hepatocarcinogenesis. Jpn J Cancer Res. 1988 May;79(5):556–572. doi: 10.1111/j.1349-7006.1988.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk D., Karger A., Lipp H. P., Bock K. W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and ethinylestradiol as co-mitogens in cultured rat hepatocytes. Carcinogenesis. 1992 Mar;13(3):453–456. doi: 10.1093/carcin/13.3.453. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R. Tumor promotion in the liver. Arch Toxicol. 1985 Aug;57(3):147–158. doi: 10.1007/BF00290879. [DOI] [PubMed] [Google Scholar]
- Umbreit T. H., Gallo M. A. Physiological implications of estrogen receptor modulation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 1988 Jul;42(1):5–14. doi: 10.1016/0378-4274(88)90097-5. [DOI] [PubMed] [Google Scholar]
- Wassom J. S., Huff J. E., Loprieno N. A review of the genetic toxicology of chlorinated dibenzo-p-dioxins. Mutat Res. 1977;47(3-4):141–160. doi: 10.1016/0165-1110(77)90001-x. [DOI] [PubMed] [Google Scholar]
- Williams G. M. The significance of chemically-induced hepatocellular altered foci in rat liver and application to carcinogen detection. Toxicol Pathol. 1989;17(4 Pt 1):663–674. doi: 10.1177/0192623389017004111. [DOI] [PubMed] [Google Scholar]
- Xu Y. H., Campbell H. A., Sattler G. L., Hendrich S., Maronpot R., Sato K., Pitot H. C. Quantitative stereological analysis of the effects of age and sex on multistage hepatocarcinogenesis in the rat by use of four cytochemical markers. Cancer Res. 1990 Feb 1;50(3):472–479. [PubMed] [Google Scholar]