Abstract
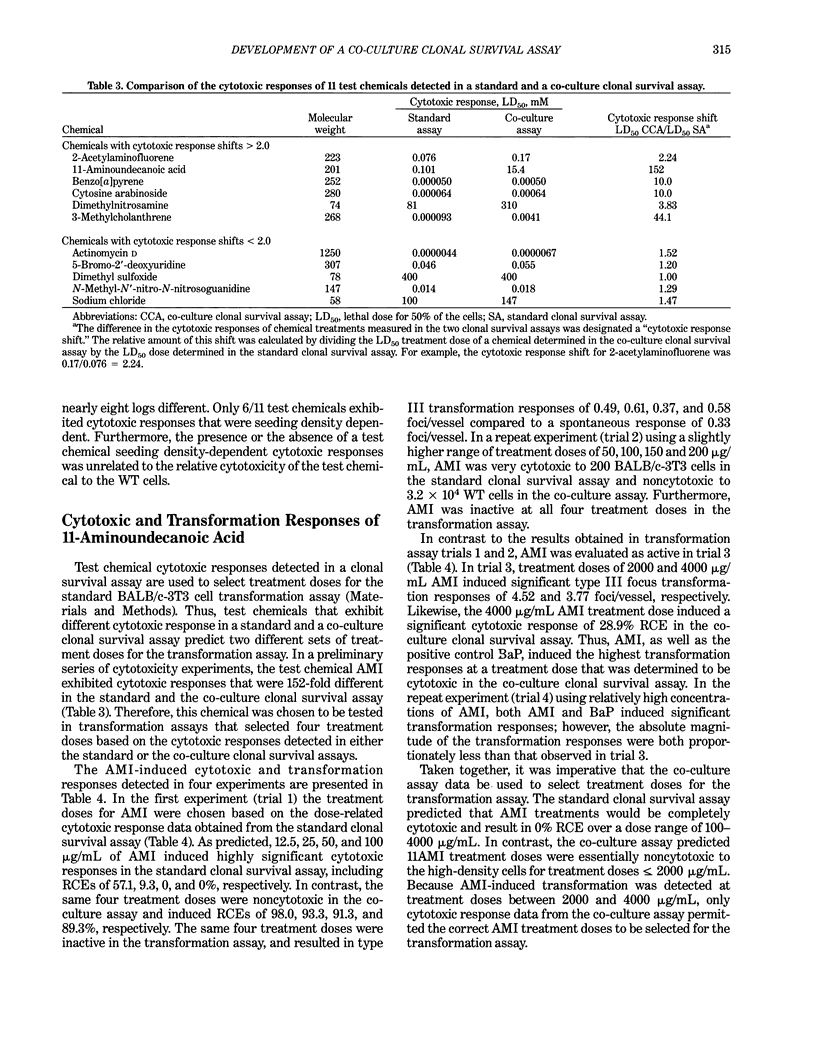
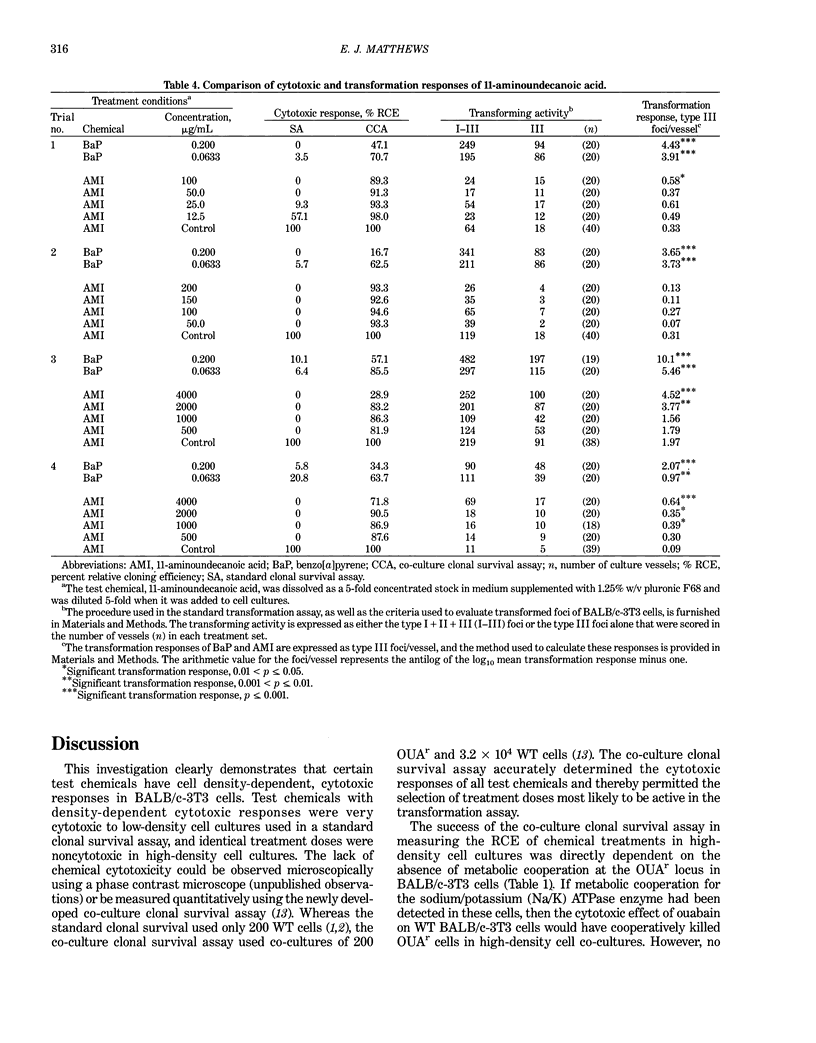
A co-culture clonal survival assay was developed to measure the cytotoxicity of test chemical treatments to BALB/c-3T3 cells because the standard clonal survival assay using 200 wild type (WT) cells frequently overestimates chemical cytotoxicity when compared with identical treatment doses in high-density cultures. The assay used co-cultures of 3.2 x 10(4) WT cells, the same seeding density used in the transformation assay, and 200 ouabain resistant (OUAr) cells. After a 48-hr test chemical treatment, co-cultured cells were fed with culture medium containing 4 mM ouabain. The test chemical was cytotoxic to an equal percentage of WT and OUAr cells. Ouabain treatments killed the remaining WT cells. Thus, OUAr cells surviving the test chemical treatment measured the relative cloning efficiency (RCE) of all treated cells in the high-density cell co-culture. The co-culture assay succeeded because metabolic cooperation at the OUAr locus was not detected in BALB/c-3T3 cells. Five chemicals induced comparable cytotoxic responses in both assays, including actinomycin D, 5-bromo-2'-deoxyuridine, N'-methyl-N-nitro-N'-nitrosoguanidine, dimethyl sulfoxide and sodium chloride. In contrast, chemical cytotoxic responses detected in the standard and co-culture assays differed by > or = 10-fold for 11-aminoundecanoic acid, benzo[a]pyrene, cytosine arabinoside, and 3-methyl-cholanthrene and differed by > 2-fold for 2-acetylaminofluorene and dimethylnitrosamine. Detection of 11-aminoundecanoic acid-induced transformation was shown to be dependent on selecting treatment doses from the co-culture assay data. Thus, this method permits more accurate assessment of both chemical-induced cytotoxicity and transformation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Tennant R. W. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat Res. 1988 Jan;204(1):17–115. doi: 10.1016/0165-1218(88)90114-0. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Galloway S. M., Armstrong M. J., Reuben C., Colman S., Brown B., Cannon C., Bloom A. D., Nakamura F., Ahmed M., Duk S. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ Mol Mutagen. 1987;10 (Suppl 10):1–175. doi: 10.1002/em.2850100502. [DOI] [PubMed] [Google Scholar]
- Galloway S. M., Bloom A. D., Resnick M., Margolin B. H., Nakamura F., Archer P., Zeiger E. Development of a standard protocol for in vitro cytogenetic testing with Chinese hamster ovary cells: comparison of results for 22 compounds in two laboratories. Environ Mutagen. 1985;7(1):1–51. doi: 10.1002/em.2860070102. [DOI] [PubMed] [Google Scholar]
- Hatch G. G., Anderson T. M., Lubet R. A., Kouri R. E., Putman D. L., Cameron J. W., Nims R. W., Most B., Spalding J. W., Tennant R. W. Chemical enhancement of SA7 virus transformation of hamster embryo cells: evaluation by interlaboratory testing of diverse chemicals. Environ Mutagen. 1986;8(4):515–531. doi: 10.1002/em.2860080404. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from BALB-3T3. Int J Cancer. 1973 Sep 15;12(2):463–473. doi: 10.1002/ijc.2910120217. [DOI] [PubMed] [Google Scholar]
- Kakunaga T., Crow J. D. Cell variants showing differential susceptibility to ultraviolet light--induced transformation. Science. 1980 Jul 25;209(4455):505–507. doi: 10.1126/science.7394516. [DOI] [PubMed] [Google Scholar]
- Landolph J. R., Telfer N., Heidelberger C. Further evidence that ouabain-resistant variants induced by chemical carcinogens in transformable C3H/10T1/2 Cl 8 mouse fibroblasts are mutants. Mutat Res. 1980 Sep;72(2):295–310. doi: 10.1016/0027-5107(80)90044-5. [DOI] [PubMed] [Google Scholar]
- Lo K. Y., Kakunaga T. Similarities in the formation and removal of covalent DNA adducts in benzo(a)pyrene-treated BALB/3T3 variant cells with different induced transformation frequencies. Cancer Res. 1982 Jul;42(7):2644–2650. [PubMed] [Google Scholar]
- Malcolm A. R., Mills L. J., Trosko J. E. Effects of ethanol, phenol, formaldehyde, and selected metabolites on metabolic cooperation between Chinese hamster V79 lung fibroblasts. Carcinog Compr Surv. 1985;8:305–318. [PubMed] [Google Scholar]
- Matthews E. J., Spalding J. W., Tennant R. W. Transformation of BALB/c-3T3 cells: IV. Rank-ordered potency of 24 chemical responses detected in a sensitive new assay procedure. Environ Health Perspect. 1993 Jul;101 (Suppl 2):319–345. doi: 10.1289/ehp.93101s2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. J. Transformation of BALB/c-3T3 cells: II. Investigation of experimental parameters that influence detection of benzo[a]pyrene-induced transformation. Environ Health Perspect. 1993 Jul;101 (Suppl 2):293–310. doi: 10.1289/ehp.93101s2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. B., Brown A., Cattanach P., Edwards I., McBride D., Riach C., Caspary W. J. Responses of the L5178Y tk+/tk- mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen. 1988;12(1):85–154. doi: 10.1002/em.2860120111. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Yamasaki H., Enomoto T., Shiba Y., Kanno Y., Kakunaga T. Intercellular communication capacity as a possible determinant of transformation sensitivity of BALB/c 3T3 clonal cells. Cancer Res. 1985 Feb;45(2):637–641. [PubMed] [Google Scholar]
- Yamasaki H., Katoh F. Further evidence for the involvement of gap-junctional intercellular communication in induction and maintenance of transformed foci in BALB/c 3T3 cells. Cancer Res. 1988 Jun 15;48(12):3490–3495. [PubMed] [Google Scholar]


