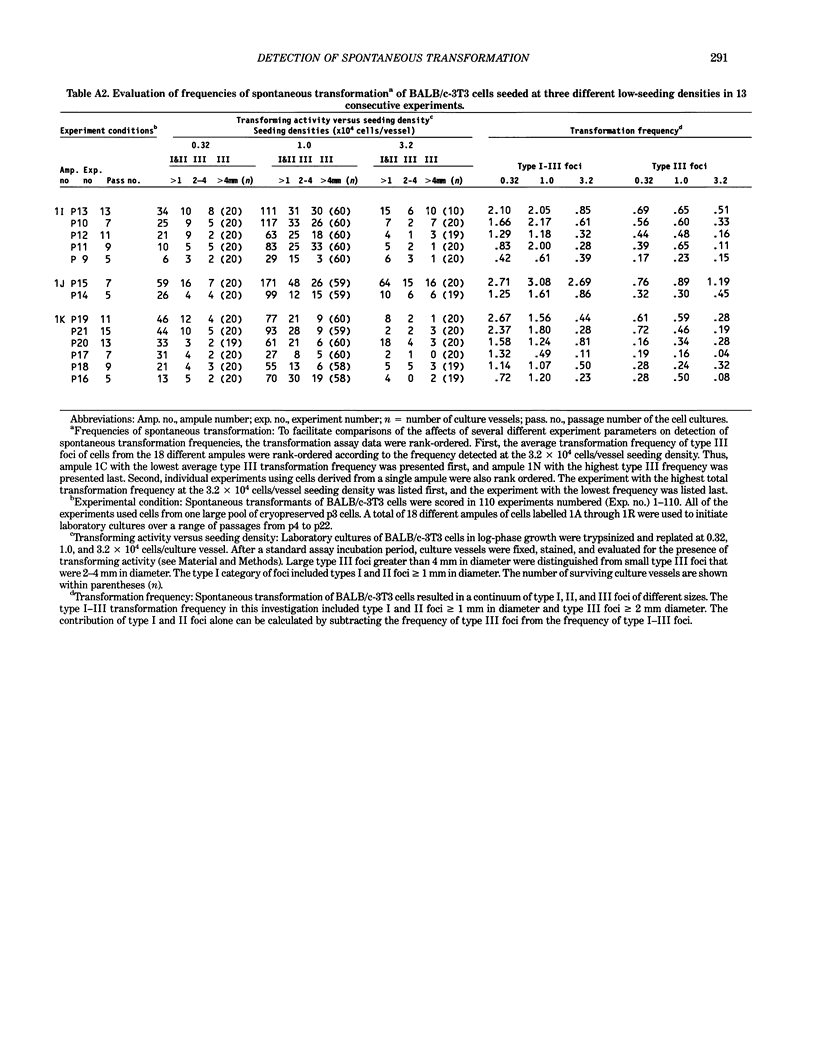
Abstract
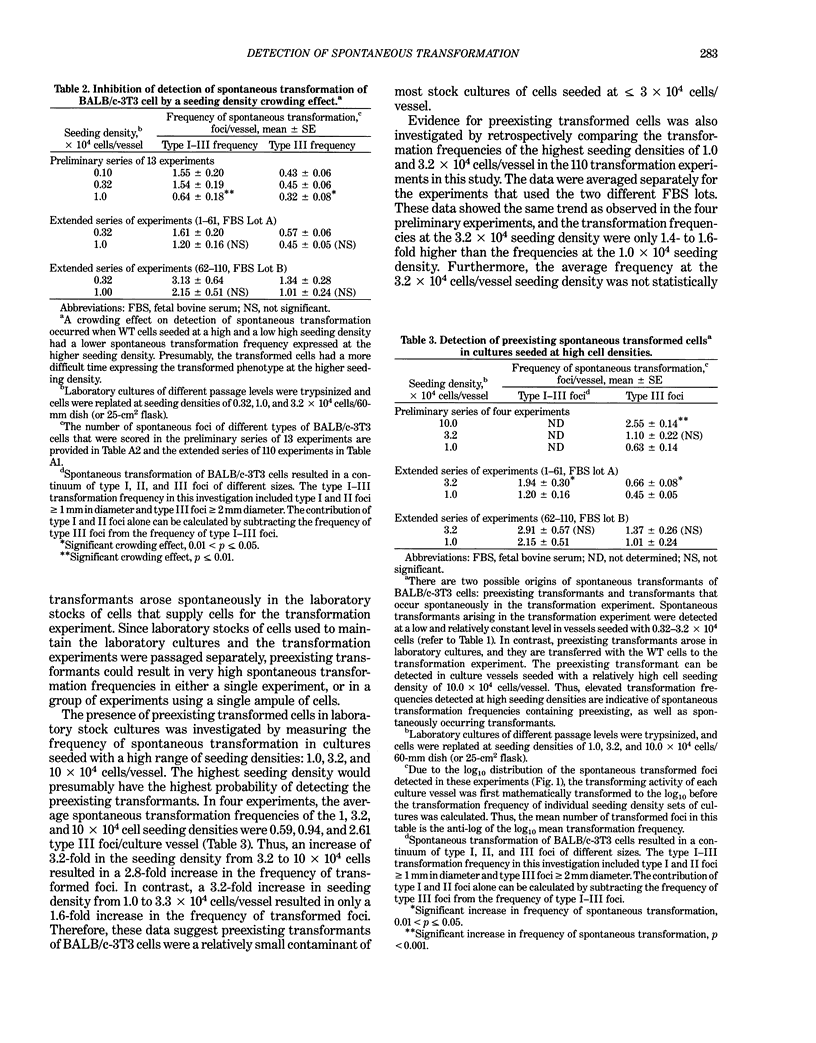
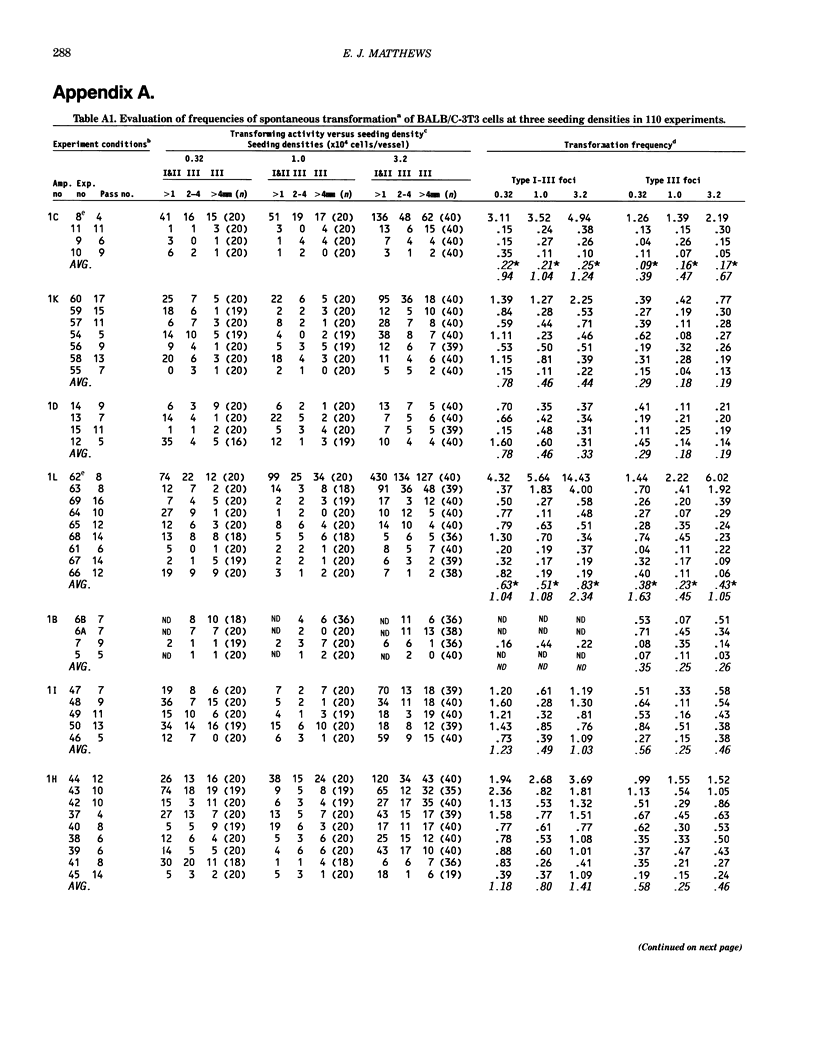
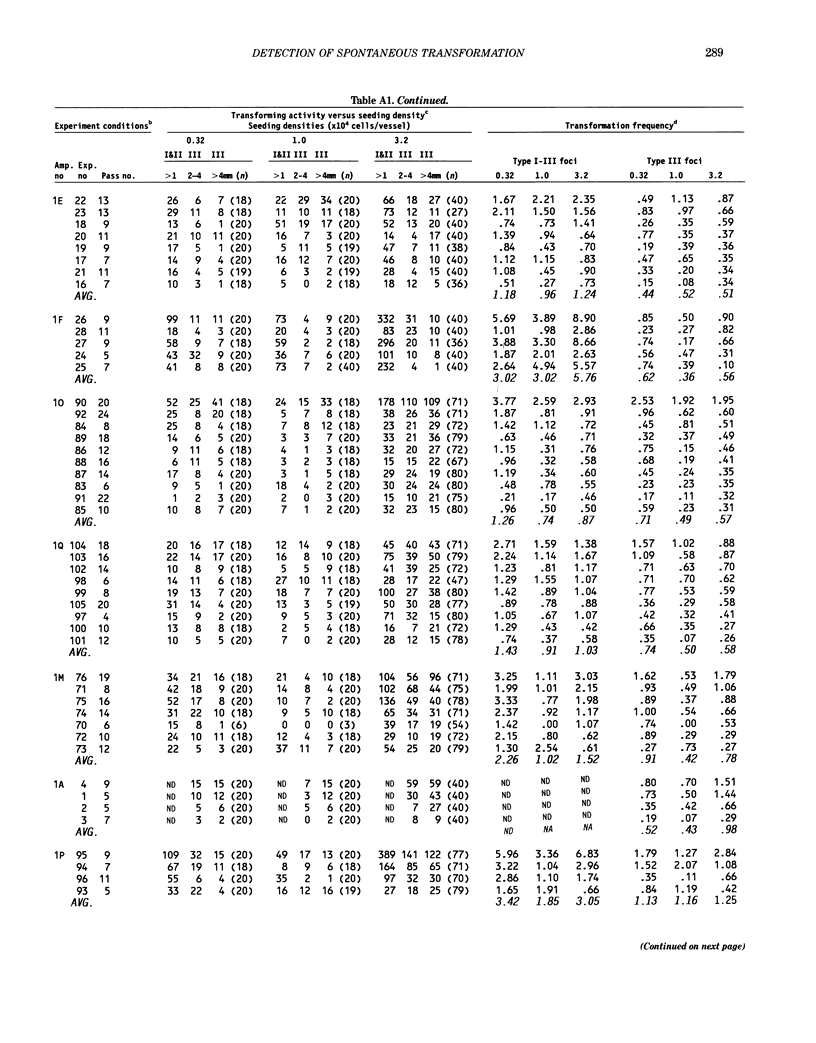
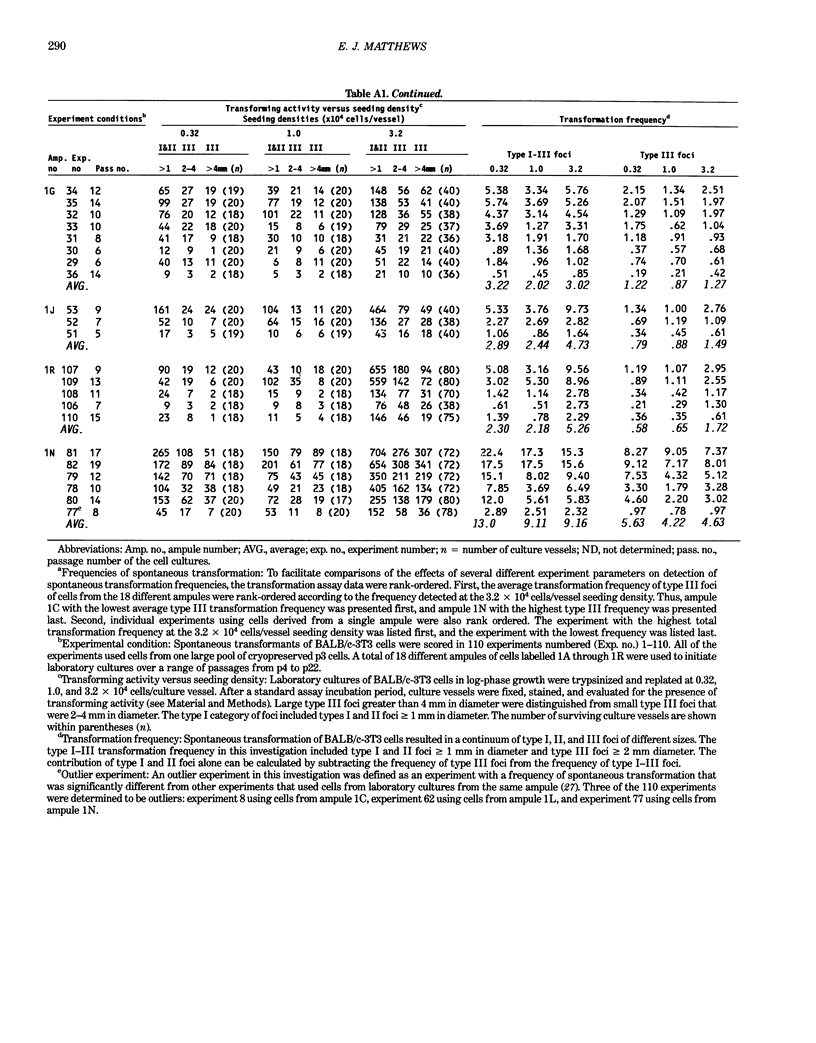
The frequency of spontaneous morphological transformation is an important variable in measuring chemical-induced transformation in BALB/c-3T3 clone A-31-1-13 cell cultures. Data from 110 experiments, which included benzo[a]pyrene control groups and other chemical treatment groups, were analyzed for factors that influenced spontaneous transformation. Spontaneous transformants demonstrated a continuum of morphological variants (type I, II, and III foci) that fit a normal distribution if converted to log10. The magnitude of transformation depended on the ampule of cryopreserved cells and the serum lot. Although the average frequency was approximately 0.71 x 10(-6) (type III foci/cell that survived and proliferated to confluence), the absolute number of foci/vessel increased in proportion to the surface area of the culture vessel. Thus, the frequency of spontaneous transformation was directly related to the cumulative number of mitoses that occurred in forming the contact-inhibited monolayer. These data are consistent with a hypothesis that spontaneous transformation in BALB/c-3T3 cells is a mutational event or some other single-step phenomenon.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertram J. S. Effects of serum concentration on the expression of carcinogen-induced transformation in the C3H/10T1/2 CL8 cell line. Cancer Res. 1977 Feb;37(2):514–523. [PubMed] [Google Scholar]
- Casto B. C., Miyagi M., Meyers J., Di Paolo J. A. Increased integration of viral genome following chemical and viral treatment of hamster embryo cells. Chem Biol Interact. 1979 May;25(2-3):255–269. doi: 10.1016/0009-2797(79)90050-4. [DOI] [PubMed] [Google Scholar]
- Casto B. C., Pieczynski W. J., DiPaolo J. A. Enhancement of adenovirus transformation by pretreatment of hamster cells with carcinogenic polycyclic hydrocarbons. Cancer Res. 1973 Apr;33(4):819–824. [PubMed] [Google Scholar]
- DiPaolo J. A. Quantitative in vitro transformation of Syrian golden hamster embryo cells with the use of frozen stored cells. J Natl Cancer Inst. 1980 Jun;64(6):1485–1489. doi: 10.1093/jnci/64.6.1485. [DOI] [PubMed] [Google Scholar]
- Dunkel V. C., Pienta R. J., Sivak A., Traul K. A. Comparative neoplastic transformation responses of Balb/3T3 cells, Syrian hamster embryo cells, and Rauscher murine leukemia virus-infected Fischer 344 rat embryo cells to chemical compounds. J Natl Cancer Inst. 1981 Dec;67(6):1303–1312. [PubMed] [Google Scholar]
- Frazelle J. H., Abernethy D. J., Boreiko C. J. Factors influencing the promotion of transformation in chemically-initiated C3H/10T1/2 Cl 8 mouse embryo fibroblasts. Carcinogenesis. 1983;4(6):709–715. doi: 10.1093/carcin/4.6.709. [DOI] [PubMed] [Google Scholar]
- Grisham J. W., Smith G. J., Lee L. W., Bentley K. S., Fatteh M. V. Induction of foci of morphologically transformed cells in synchronized populations of 10T1/2 cells by N-methyl-N'-nitro-N-nitrosoguanidine and the effect of spontaneous transformation on calculated transformation frequency. Cancer Res. 1988 Nov 1;48(21):5977–5983. [PubMed] [Google Scholar]
- Grisham J. W., Smith G. J., Lee L. W., Bentley K. S., Fatteh M. V. Spontaneous formation of foci of morphologically transformed cells in populations of C3H 10T1/2 (clone 8) cells. Cancer Res. 1988 Nov 1;48(21):5969–5976. [PubMed] [Google Scholar]
- Haber D. A., Fox D. A., Dynan W. S., Thilly W. G. Cell density dependence of focus formation in the C3H/10T1/2 transformation assay. Cancer Res. 1977 Jun;37(6):1644–1648. [PubMed] [Google Scholar]
- Heidelberger C., Freeman A. E., Pienta R. J., Sivak A., Bertram J. S., Casto B. C., Dunkel V. C., Francis M. W., Kakunaga T., Little J. B. Cell transformation by chemical agents--a review and analysis of the literature. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983 Apr;114(3):283–385. doi: 10.1016/0165-1110(83)90036-2. [DOI] [PubMed] [Google Scholar]
- Heidelberger C., Landolph J. R., Fournier R. E., Fernandez A., Peterson A. R. Genetic and probability aspects of cell transformation by chemical carcinogens. Prog Nucleic Acid Res Mol Biol. 1983;29:87–98. doi: 10.1016/s0079-6603(08)60433-x. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from BALB-3T3. Int J Cancer. 1973 Sep 15;12(2):463–473. doi: 10.1002/ijc.2910120217. [DOI] [PubMed] [Google Scholar]
- Kakunaga T., Crow J. D. Cell variants showing differential susceptibility to ultraviolet light--induced transformation. Science. 1980 Jul 25;209(4455):505–507. doi: 10.1126/science.7394516. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K. Y., Kakunaga T. Similarities in the formation and removal of covalent DNA adducts in benzo(a)pyrene-treated BALB/3T3 variant cells with different induced transformation frequencies. Cancer Res. 1982 Jul;42(7):2644–2650. [PubMed] [Google Scholar]
- Matthews E. J., Spalding J. W., Tennant R. W. Transformation of BALB/c-3T3 cells: IV. Rank-ordered potency of 24 chemical responses detected in a sensitive new assay procedure. Environ Health Perspect. 1993 Jul;101 (Suppl 2):319–345. doi: 10.1289/ehp.93101s2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. J., Spalding J. W., Tennant R. W. Transformation of BALB/c-3T3 cells: V. Transformation responses of 168 chemicals compared with mutagenicity in Salmonella and carcinogenicity in rodent bioassays. Environ Health Perspect. 1993 Jul;101 (Suppl 2):347–482. doi: 10.1289/ehp.93101s2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. J. Transformation of BALB/c-3T3 cells: II. Investigation of experimental parameters that influence detection of benzo[a]pyrene-induced transformation. Environ Health Perspect. 1993 Jul;101 (Suppl 2):293–310. doi: 10.1289/ehp.93101s2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S., Brankow D. W., Heidelberger C. Two-stage chemical oncogenesis in cultures of C3H/10T1/2 cells. Cancer Res. 1976 Jul;36(7 Pt 1):2254–2260. [PubMed] [Google Scholar]
- Nakazawa H., Aguelon A. M., Yamasaki H. Relationship between chemically induced Ha-ras mutation and transformation of BALB/c 3T3 cells: evidence for chemical-specific activation and cell type-specific recruitment of oncogene in transformation. Mol Carcinog. 1990;3(4):202–209. doi: 10.1002/mc.2940030407. [DOI] [PubMed] [Google Scholar]
- Nesnow S., Garland H., Curtis G. Improved transformation of C3H10T1/2CL8 cells by direct- and indirect-acting carcinogens. Carcinogenesis. 1982;3(4):377–380. doi: 10.1093/carcin/3.4.377. [DOI] [PubMed] [Google Scholar]
- Oshiro Y., Balwierz P. S., Piper C. E. Selection of fetal bovine serum for use in the C3H/10T 1/2 CL8 cell transformation assay system. Environ Mutagen. 1982;4(5):569–574. doi: 10.1002/em.2860040508. [DOI] [PubMed] [Google Scholar]
- Pienta R. J., Poiley J. A., Lebherz W. B., 3rd Morphological transformation of early passage golden Syrian hamster embryo cells derived from cryopreserved primary cultures as a reliable in vitro bioassay for identifying diverse carcinogens. Int J Cancer. 1977 May 15;19(5):642–655. doi: 10.1002/ijc.2910190508. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Rubin A. L., Arnstein P., Rubin H. Physiological induction and reversal of focus formation and tumorigenicity in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10005–10009. doi: 10.1073/pnas.87.24.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivak A., Van Duuren B. L. Phenotypic expression of transformation: induction in cell culture by a phorbol ester. Science. 1967 Sep 22;157(3795):1443–1444. doi: 10.1126/science.157.3795.1443. [DOI] [PubMed] [Google Scholar]
- Umeda M., Ono T. Cellular mechanisms of transformation of BALB/3T3 A31-1-1 cells [corrected] by gamma-irradiation. Jpn J Cancer Res. 1986 Mar;77(3):255–263. [PubMed] [Google Scholar]
- Whorton E. B., Jr, Ward J. B., Jr, Morris D. L. Experimental design and statistical analysis considerations for in vitro mammalian cell transformation assays with BALB/3T3 cells. Environ Mutagen. 1982;4(5):595–603. doi: 10.1002/em.2860040511. [DOI] [PubMed] [Google Scholar]


