Abstract
Tuberculosis is the seventh leading cause of morbidity and mortality in the world, with eight million cases per year. Animal and human studies demonstrate an enrichment of CD4 cells at sites of disease, with a more favorable clinical course when there is a Th1 response with the presence of gamma interferon (IFN-γ). We previously treated patients who had multidrug-resistant tuberculosis with recombinant IFN-γ (rIFN-γ) in aerosol form and were able to convert smear-positive cases to smear negative with 12 treatments over 1 month. We hypothesized that rIFN-γ would induce signal transducer and activator of transcription (STAT) and interferon regulatory factor (IRF) binding activity in alveolar macrophages (AM). AM treated in vitro showed clear upregulation of STAT-1 and IRF-1 by rIFN-γ. STAT-1 was not activated and IRF-1 was only weakly induced after 1 day of infection by Mycobacterium tuberculosis TN913. In bronchoalveolar lavage (BAL) cells obtained from 10 of 10 tuberculosis patients 10 ± 2 days post-antituberculosis treatment, there was no detectable STAT-1 or IRF-1 DNA-binding activity. After 4 weeks of treatment with rIFN-γ aerosol in addition to the antituberculosis drugs, 10 of 10 patients had increased STAT-1, IRF-1, and/or IRF-9 DNA-binding activity in BAL cells from lung segments shown radiographically to be involved and in those shown to be uninvolved. Symptoms and chest radiographs improved, and amounts of macrophage inflammatory cytokines and human immunodeficiency virus type 1 (HIV-1) viral loads (in five of five HIV-1-coinfected patients) declined in the second BAL specimens. rIFN-γ aerosol induces signal transduction and gene expression in BAL cells and should be evaluated for efficacy in a randomized, controlled clinical trial.
Mycobacterium tuberculosis infects one-third of the world's population, causes eight million active cases of tuberculosis per year, and ranks seventh in terms of global morbidity and mortality (12, 31). Challenges in conquering this disease include the need to prolong chemotherapy for 6 months to eliminate persistent organisms and the need to prevent the lung destruction, bronchiectasis, and fibrosis seen in advanced cases (2). Patients with bilateral pulmonary tuberculosis, cavitary disease, and persistently positive sputum smears have special risks of treatment failure and/or relapse. We hypothesized that gamma interferon (IFN-γ) and the CD4 Th1 phenotype would stimulate signaling molecules, activate alveolar macrophages (AM), and improve clinical outcome, perhaps reducing the necessary duration of chemotherapy and the extent of tissue destruction (39).
The rate of activation of latent tuberculosis in purified protein derivative-positive AIDS patients is 10% per year, compared to a 10% lifetime risk of progression in immunocompetent purified protein derivative-positive patients (43). Tuberculosis causes a marked increase in human immunodeficiency virus type 1 (HIV-1) replication and mutation in the involved lung segments (32, 46). This may underlie the accelerated progression of AIDS observed in coinfected patients (50). While AIDS patients are capable of producing normal amounts of many proinflammatory cytokines in involved lung segments, they fail to produce as much IFN-γ mRNA as immunocompetent patients with tuberculosis (28). In non-HIV-infected patients, segments found radiographically to be involved show an enrichment of CD4+ cells, and in patients with minimal pulmonary tuberculosis, there is an increase in the spontaneous release of IFN-γ and in IFN-γ mRNA expression (5, 28). The presence of IFN-γ in bronchoalveolar lavage (BAL) fluid is associated with less-advanced tuberculosis, and augmentation with IFN-γ in aerosol form has been shown to be safe in normal volunteers (21). This therapy induced AM gene expression of IP-10, whereas subcutaneous administration did not (21). Previously, recombinant IFN-γ (rIFN-γ) aerosol was given three times weekly for 1 month to five pulmonary tuberculosis patients for whom second-line therapy for multidrug resistance was failing; the treatment was demonstrated to have clinical efficacy, resulting in the conversion of sputum smears from positive to negative, weight gain, and improved radiographs (6).
The binding of six-helix monomeric IFN-γ to the 90-kDa IFN-γ receptor initiates a signal transduction pathway that culminates in the activation of gene expression (reviewed in references 9 and 19). Binding occurs at the β-subunits and leads to the activation of Janus kinase 1 and 2 tyrosine kinases through auto- or transphosphorylation of kinase tyrosine residues. Janus kinase activation leads to tyrosine phosphorylation of the latent cytoplasmic signal transducer and activator of transcription 1 (STAT-1), which homodimerizes and translocates to the nucleus. There STAT-1 binds to regulatory regions of genes containing IFN-γ activation site (GAS) consensus sequences. Among the genes activated by STAT-1 binding is that for the transcription factor interferon regulatory factor 1 (IRF-1). This factor, in turn, can activate a large number of genes by binding to the interferon-stimulated response element (ISRE) (36). IRF-9, another member of the IRF family, is also newly synthesized in cells stimulated by IFN-γ (29, 47). Unlike IRF-1, IRF-9 gene expression is also dependent on new protein synthesis, through mechanisms that are still being defined (17, 40, 49).
In the present study, we investigated the AM response to rIFN-γ in order to ascertain the effects on signaling molecules in vitro and in vivo. We found that treatment with rIFN-γ did activate signal transduction pathways and gene expression, and in tuberculosis patients we found increased DNA-binding activity for STAT-1 and IRF family members, including IRF-1 and IRF-9, only after the aerosol rIFN-γ was added to the antituberculosis treatment regimen.
MATERIALS AND METHODS
Study population.
The protocol for this study was approved by the Human Subjects Review Committees of New York University School of Medicine and Bellevue Hospital Center. Normal volunteers with normal chest radiographs, spirometry results, and physical examinations were recruited. Eleven patients with active pulmonary tuberculosis participated. HIV-1 testing was done on all patients by using enzyme-linked immunosorbent assays, and results were confirmed by Western blotting. HIV-1-infected patients were not on antiretroviral therapy. For all patients, M. tuberculosis isolates were recovered from sputum cultures, and 10 of 11 culture isolates were sensitive to first-line antituberculosis medication. All patients had their symptoms reviewed and had chest computerized tomography scans and active evaluations relative to rIFN-γ aerosol treatment. Patients received 500 μg of rIFN-γ (Intermune) mixed with 3 ml of normal saline via a Respirgard nebulizer three times a week for 4 weeks in addition to their antituberculosis medications.
BAL.
BAL was performed with a flexible fiber-optic bronchoscope, and patients were given local anesthesia (Xylocaine). It has been established that BAL cells are representative of inflammatory and immune cells from the lung parenchyma in various circumstances, including during tuberculosis (18, 28). BAL was first performed in 10 patients 10 ± 2 days after the start of treatment with antituberculosis drugs; in one patient with multidrug-resistant tuberculosis, BAL was done after 12 months of second-line therapy. A follow-up BAL was performed approximately 1 h or 1 day after the final aerosol IFN-γ treatment, as previously described (6). BAL was done on six patients, including three of five HIV-1-infected tuberculosis patients, 1 h after treatment with aerosol rIFN-γ. It was done on four patients, including two of five HIV-1-infected tuberculosis patients, 1 day after treatment with aerosol rIFN-γ. The change in follow-up time from 1 day to 1 h after was made for the convenience of the patients (allowing them to avoid another trip to the hospital). Normal saline (6 50-ml aliquots) was instilled and suctioned sequentially from two or three sites (including sites shown radiographically to be involved). The recovered fluid was filtered through sterile gauze. A total cell count was done in a hematocytometer, cell differentials were performed on cytocentrifuge slides stained with Diff-Quick, and 500 cells were counted. Cell viability was determined by trypan blue exclusion, and in all cases, recovered cells were >90% viable.
Immunoassays.
All BAL fluids were concentrated (10×) with Centriprep-10 filters (Amicon, Beverly, Mass.) for the measurement of cytokines. Tumor necrosis factor alpha (TNF-α), IFN-γ, and interleukin-15 (IL-15) enzyme-linked immunosorbent assay kits were purchased and used according to the recommendations of the manufacturer (R & D, Minneapolis, Minn.). Protein concentrations for BAL fluids were determined with Bio-Rad Bradford assay reagent. HIV-1 viral loads were quantitated in BAL fluids from HIV-1-infected patients by reverse transcription-PCR assay (Ultrasensitive; Roche Molecular Systems, Pleasanton, Calif.) as previously described (16).
Protein extracts and EMSA.
All procedures with BAL cells were performed in a biosafety level 3 laboratory. BAL cells from healthy volunteers were uninfected or infected by M. tuberculosis TN913 for 1 day in vitro and then either unstimulated or stimulated with rIFN-γ (5 ng/ml) for the final 2 h prior to the preparation of protein extracts as previously described (48). If so indicated (see Fig. 1 and 2), medium containing nonadherent cells was pooled with a phosphate-buffered saline wash of the monolayer, and extracts were then prepared separately from the nonadherent cells, principally lymphocytes, and the adherent cells, principally AM. BAL cells from patients were either put at 0 to 4°C as soon as possible after lavage or cultured in RPMI 1640 (BioWhittaker) plus 10% fetal bovine serum (HyClone) at 37°C and 5% CO2 for 1 h so that nonadherent cells could be separated from adherent cells as described above. In either case, extraction was started within 2 h after the completion of BAL. An electrophoretic mobility shift assay (EMSA) was performed with approximately 10 μg of extract protein (2 to 3 μl of extract) as previously described (48). The ISRE oligonucleotide (CTCGGGAAAGGGAAACCGAAACTGAAGCC) and its complement, synthesized with BamHI cohesive termini at the 5′ end of each strand, span from −117 to −89 of the ISG15 promoter (38). The ISRE homology is shown in bold. The GAS oligonucleotide (TACAACAGCCTGATTTCCCCGAAATGACGGC) and its complement, synthesized with HindIII cohesive termini at the 5′ end of each strand, span from −137 to −107 of the IRF-1 promoter (35). The GAS homology is shown in bold. The nonspecific oligonucleotide (CTCTCTGCAAGGGTCATCAGTAC) and its complement, synthesized with HindIII cohesive termini at the 5′ end of each strand, include the distal hepatocyte nuclear factor 4 site from the transthyretin promoter (44). Rabbit polyclonal anti-IRF-1 antiserum was raised against the human protein purified from HeLa cells (36). Rabbit polyclonal anti-STAT-1 antiserum was a gift of Chris Schindler (42). Rabbit polyclonal anti-IRF-9 antiserum was a gift of David Levy (47). An irrelevant immune rabbit antiserum was used as a control for the specificities of the anti-IRF and anti-STAT sera. If indicated (see Fig. 2, 3, and 4), excess unlabeled oligonucleotide was included in the binding reaction, or 2.5 μl of 1× binding buffer containing 0.5 μl of antiserum or antibody was added. Radioactivity in protein-DNA complexes was visualized and quantified with a PhosphorImager and ImageQuant software (Molecular Dynamics).
FIG. 1.
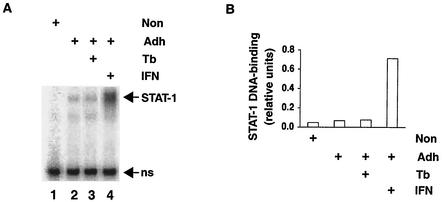
STAT-1 DNA-binding activity in AM after stimulation with rIFN-γ or infection by M. tuberculosis in vitro. (A) BAL cells were obtained from healthy volunteers and stimulated with IFN-γ or infected by M. tuberculosis in vitro (Tb) as indicated. Protein extracts were prepared from nonadherent (Non) and adherent (Adh) cells, and STAT-1 DNA-binding activity was determined by EMSA. The specific complexes formed between STAT-1 and a GAS oligonucleotide probe are indicated (STAT-1). The STAT-1-GAS complex has been identified by its characteristic mobility, sequence specificity, and specific reaction with anti-STAT-1 antiserum (data not shown). A nonspecific complex (ns) serves as an internal standard for recovery of protein and gel loading. (B) STAT-1 activity was quantified relative to that of the nonspecific complex.
FIG. 2.
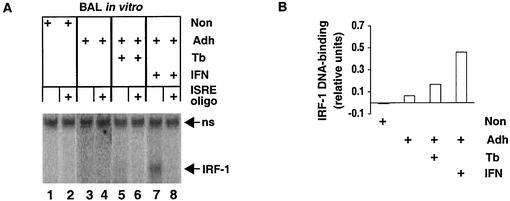
IRF-1 DNA-binding activity in AM after stimulation with rIFN-γ or infection by M. tuberculosis in vitro. (A) BAL cells were obtained from healthy volunteers and stimulated with IFN-γ or infected by M. tuberculosis in vitro (Tb) as indicated. Protein extracts were prepared from nonadherent (Non) and adherent (Adh) cells, and IRF-1 DNA-binding activity was determined by EMSA in the presence of nonspecific or specific (ISRE oligo) excess unlabeled oligonucleotide competitor. The specific complexes formed between IRF-1 and an ISRE oligonucleotide probe are indicated (IRF-1). The complex was identified by specific reaction with anti-IRF-1 antiserum (data not shown). A nonspecific complex (ns) serves as an internal standard for recovery of protein and gel loading. (B) IRF-1 activity was quantified relative to that of the nonspecific complex.
FIG. 3.
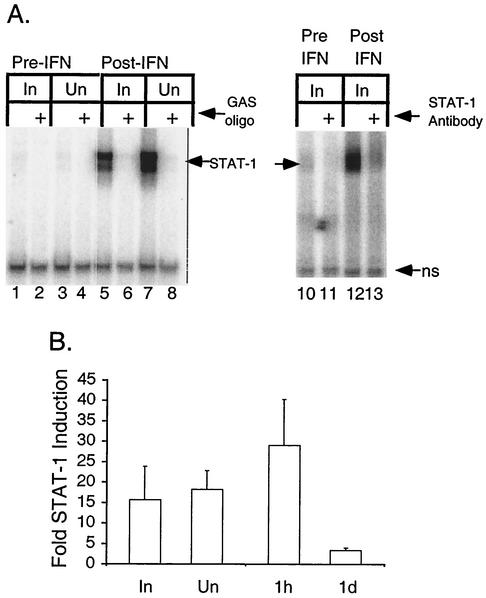
STAT-1 DNA-binding activity in BAL cells before and after aerosolized rIFN-γ therapy. (A) Protein extracts were prepared from BAL cells obtained from lung segments shown radiographically to be involved (In) or uninvolved (Un) before (Pre-IFN) or at the conclusion of (Post-IFN) treatment with aerosolized rIFN-γ. The specific complexes formed between STAT-1 and a GAS oligonucleotide probe, as detected by EMSA, are indicated (STAT-1). A nonspecific complex (ns) serves as an internal standard for recovery of protein and gel loading. Left panel: unlabeled nonspecific oligonucleotide or GAS oligonucleotide (GAS oligo) was included in the assay. Right panel: nonspecific antibody or anti-STAT-1 antibody (STAT-1 Antibody) was included in the assay. (B) STAT-1 DNA-binding activity was quantified for BAL cell extracts from 10 patients. The average fold increases in the levels of induction posttherapy relative to the levels pretherapy are shown. Cells were obtained from uninvolved or involved segments at 1 h and 1 day (1d) after the final aerosolized rIFN-γ treatment. Error bars indicate standard errors of the means.
FIG. 4.
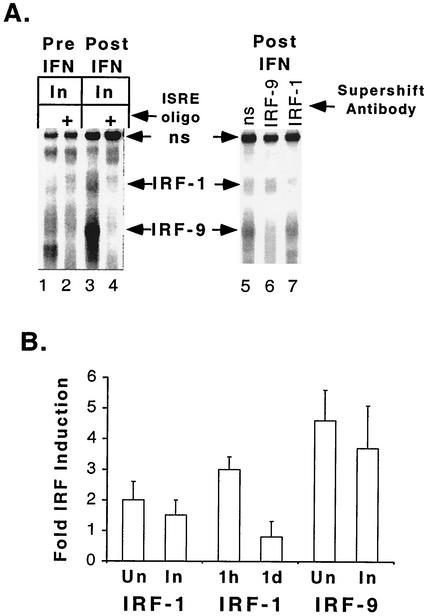
IRF-1 and IRF-9 DNA-binding activity in BAL cells before and after aerosolized IFN-γ therapy. (A) Protein extracts were prepared from BAL cells obtained from lung segments shown radiographically to be involved (In) before (Pre IFN) or at the conclusion of (Post IFN) treatment with aerosolized IFN-γ. The specific complexes formed between IRFs and an ISRE oligonucleotide probe, as detected by EMSA, are indicated (IRF-1 and IRF-9, with arrows). A nonspecific complex (ns, with arrows) serves as an internal standard for recovery of protein and gel loading. Left panel: unlabeled nonspecific oligonucleotide or ISRE oligonucleotide (ISRE oligo) was included in the assay. Right panel: nonspecific antibody (ns), anti-IRF-9 antibody (IRF-9), or anti-IRF-1 antibody (IRF-1) was included in the assay. (B) IRF-1 and IRF-9 DNA-binding activities were quantified for BAL cell extracts from nine and six patients, respectively. The average fold increases in the levels of induction posttherapy relative to the levels pretherapy are shown. Cells were obtained from uninvolved (Un) or involved segments at 1 h and 1 day (1d) after the final aerosolized rIFN-γ treatment. Error bars indicate standard errors of the means.
Biostatistics.
The Wilcoxon signed rank test was used for comparisons of BAL parameters, and a P value of ≤0.05 was chosen to indicate statistical significance.
RESULTS
Response of AM to IFN-γ in vitro.
First we evaluated the response of AM to IFN-γ in vitro. AM recovered from healthy volunteers by BAL were infected by M. tuberculosis or stimulated with rIFN-γ. The occurrence of signal transduction was monitored by measuring STAT-1 homodimer DNA-binding activity (Fig. 1A). In contrast to nonadherent cells (lane 1), adherent cells, primarily AM, had a detectable basal level of STAT-1 homodimers (lane 2). In vitro infection by M. tuberculosis had little or no effect (lane 3). After stimulation with rIFN-γ for 2 h, STAT-1 homodimer DNA-binding activity was much greater (lane 4). The increase over that in unstimulated cells was approximately 10-fold (Fig. 1B). Infection did not alter rIFN-γ stimulation of STAT-1 homodimer DNA-binding activity in AM (Y. Qiao, S. Prabhakar, M. Weiden, and R. Pine, unpublished observations), as was also found for peripheral blood monocyte-derived macrophages (45).
To determine if signal transduction was functional in the BAL cells, the induction of IRF DNA-binding activity was assayed (Fig. 2A). Minimal or no induction of IRF-9 was observed after 2 h of IFN-γ stimulation. No IRF-1 activity was apparent in either nonadherent or adherent cells prior to infection or stimulation (lanes 1 and 3). Infection for 1 day led to a slight induction of IRF-1 (lane 5), consistent with the greater level of induction observed 3 days postinfection (37). rIFN-γ stimulation clearly induced IRF-1 (lane 7). The minimal IRF-1 level in unstimulated, uninfected cells and the induced IRF-1 levels in cells that were infected or stimulated are most apparent upon comparison with results from assays that included specific competitor oligonucleotide (lanes 2, 4, 6, and 8). There was no effect of infection on rIFN-γ stimulation of IRF-1 (Qiao et al., unpublished), consistent with the lack of effect on STAT-1 (Fig. 1). The level of IRF-1 in stimulated AM increased approximately eightfold over that in unstimulated cells (Fig. 1B). These data demonstrate that AM exhibit typical molecular responses to IFN-γ and suggest that they are likely to be affected in situ by aerosol rIFN-γ administered to tuberculosis patients.
Effect of aerosol rIFN-γ on STAT-1 and IRFs in vivo.
There was no STAT-1 DNA-binding activity in BAL cells from uninvolved or involved lobes prior to the commencement of therapy with rIFN-γ aerosol, which followed a mean of 10 ± 2 days of antituberculosis therapy, consistent with the fact that M. tuberculosis infection in vitro does not activate STAT-1 DNA binding. This was true whether, prior to extraction within 2 h after BAL, cells were cultured for 1 h at 37°C for separation into adherent and nonadherent populations or whether they were kept at 0 to 4°C. STAT-1 DNA-binding activity was induced in BAL cells from both involved and uninvolved lobes of one patient, representative of 10, after rIFN-γ aerosol treatment (Fig. 3A, lanes 5 and 7), as demonstrated by the formation of a complex between whole cell extracts and a GAS oligonucleotide probe in the presence of excess nonspecific unlabeled oligonucleotide. Excess unlabeled GAS oligonucleotide competed away the specific binding (Fig. 3A, lanes 6 and 8). The addition of nonspecific antiserum had no effect on the complex formed with the GAS oligonucleotide (Fig. 3A, lane 12), while anti-STAT-1 antibody reacted with it (Fig. 3A, lane 13), confirming that the induced DNA-binding activity in patient 5 was STAT-1. We measured an increase in the amount of STAT-1 complexes in 10 of 10 patients evaluated. Induction levels were 15- to 18-fold higher and were similar for involved and uninvolved lung segments (Fig. 3B). When STAT-1 in cells obtained 1 h or 1 day after the final rIFN-γ treatment was considered separately, the induction was found to be 29-fold or 3-fold higher, respectively, than that in the respective pretreatment cells (Fig. 3B). This difference in the levels of induction was significant (P = 0.05), and the 29-fold-higher induction was significant compared to that in the respective pretreatment cells (P = 0.03). Thus, IFN-γ signal transduction occurred in vivo in response to rIFN-γ aerosol but was not present after antituberculosis therapy alone.
We found that levels of IRF-1 and IRF-9 were also increased in extracts of BAL cells from involved segments (Fig. 4A, lane 3). The specific complexes with the labeled ISRE probe are those not detected in the presence of excess unlabeled ISRE oligonucleotide (Fig. 4A, lane 4). In another patient, we observed similar upregulation of IRF-1 and IRF-9 (Fig. 4A, lane 5). The identities of IRF-1 and IRF-9 in the complexes were demonstrated with specific antisera. Anti-IRF-9 and anti-IRF-1 antisera each reacted with different specific complexes, and the complex recognized by one antiserum did not react with the other (Fig. 4A, lanes 6 and 7). There were 2- and 1.5-fold increases in the amounts of specific IRF-1-ISRE complexes detected by EMSA of BAL cell extracts from uninvolved and involved sites, respectively, of 9 of 10 tuberculosis patients evaluated after treatment with rIFN-γ aerosol (Fig. 4B). However, analysis of induction as a function of the length of time between the final treatment with rIFN-γ and BAL showed 3-fold-higher induction of IRF-1 in cells obtained 1 h after treatment than in pretreatment cells and no induction in cells obtained 1 day after treatment (Fig. 4B). The induction of IRF-9-ISRE complexes detected by EMSA of BAL cell extracts from uninvolved and involved sites was 4.6- and 3.7-fold higher, respectively, than that in pretreatment cells for six patients from whom BAL cells were obtained 1 h after the final treatment with rIFN-γ (Fig. 4B). IRF-9 was not induced in BAL cells obtained 1 day after the final treatment with rIFN-γ (data not shown). In only one patient, who did not receive aerosol rIFN-γ, IRF-9 was detected in the extract from the first BAL cell sample, obtained 10 days after the start of antituberculosis chemotherapy (data not shown). The observed induction of IRF DNA-binding activity demonstrates that signal transduction in response to rIFN-γ aerosol was followed by gene expression.
BAL findings.
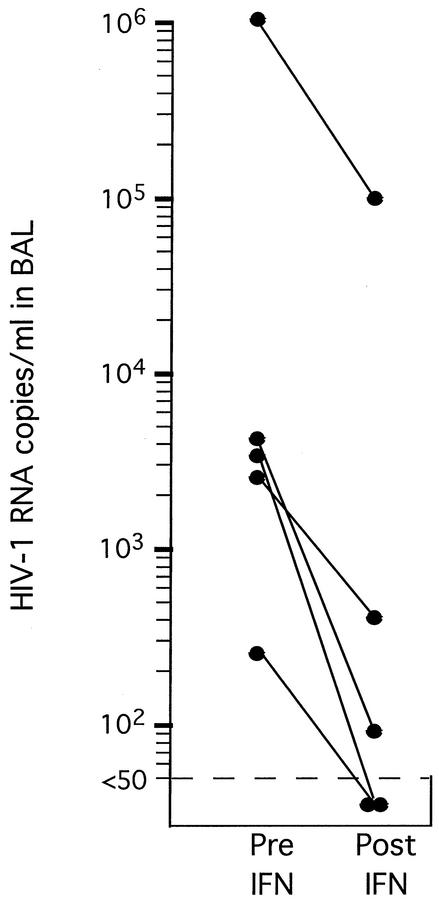
To determine what changes occurred in the lung during treatment with rIFN-γ aerosol and standard chemotherapy, BAL was performed pre- and posttreatment with rIFN-γ aerosol on lung segments shown radiographically to be involved or uninvolved. There were decreases from 34 ± 7% to 26 ± 8% in the percentage of lymphocytes and from 1,425 ± 500 to 1,132 ± 412 in the absolute number of lymphocytes per ml of BAL fluid after treatment with aerosol rIFN-γ. Viral loads in the five individuals with HIV-tuberculosis coinfection were measured in the involved lung segments pre- and posttreatment with rIFN-γ aerosol (Fig. 5). Four individuals had a decrease of greater than 1 log in HIV-1 concentration, and the fifth had a decline of almost 3 logs. One of these patients had multidrug-resistant tuberculosis, and treatment with rIFN-γ aerosol was added to the ongoing regimen of chemotherapy to which the patient had not responded.
FIG. 5.
HIV-1 concentration in BAL fluids of AIDS patients with tuberculosis after antituberculosis treatment (Pre IFN) and after treatment with aerosolized IFN-γ (Post IFN). The relative HIV-1 concentration was determined by reverse transcription-PCR. One patient had received chemotherapy for multidrug-resistant tuberculosis for 12 months prior to the start of aerosolized IFN-γ therapy, and the addition of IFN-γ was the only change in therapy.
Among cytokines released by BAL cells during 24 h in culture, declines in the mean values for TNF-α from 60 ± 40 to 5 ± 4 pg per million macrophages and in the mean values for IL-15 from 26 ± 24 to 1 ± 0.5 pg per million macrophages were observed. IL-15 has been shown to be important for IFN-γ release from NK cells (4). There was also a decline in IFN-γ levels expressed from 24-h cell culture supernatants from 6.8 ± 3.2 to 3.5 ± 1.5 pg per million lymphocytes.
Clinical outcome.
All 11 patients tolerated rIFN-γ aerosol treatment without fever, bronchospasm, or respiratory symptoms. There were nine males and two females, and their mean age was 38 ± 3 years (range, 22 to 55 years). Five were infected with HIV-1, with a mean CD4 count of 122 ± 47 cells (range, 10 to 226 cells). Most had advanced pulmonary tuberculosis; 6 of 11 had cavitary tuberculosis, 5 of 11 had bilateral infiltrates, and 2 of 11 had unilateral infiltrates. Following 1 month of rIFN-γ aerosol treatment, there was a striking decline in symptoms, with decreases in the numbers of patients experiencing each symptom as follows: fever, 4 of 11 to 1 of 11; night sweats, 7 of 11 to 3 of 11; persistent cough, 8 of 11 to 3 of 11; and sputum production (greater than 2 tablespoons per day), 6 of 11 to 0 of 11. Five of 11 patients had documented weight gain. None had hemoptysis. A review of chest radiographs before treatment revealed that a mean of 2.4 ± 0.4 lung lobes was involved. This mean declined after 1 month of therapy including rIFN-γ aerosol treatment to 2.1 ± 0.4 lung lobes. The tuberculosis patients with cavities had a mean size reduction of the cavities shown on chest radiographs from a maximal diameter of 12 ± 1.7 mm to a maximal diameter of 9 ± 2.8 mm after treatment with rIFN-γ aerosol.
DISCUSSION
We have shown that in vitro stimulation with rIFN-γ for 2 h induces STAT-1 and IRF-1 DNA-binding activity in AM (Fig. 1 and 2) and, that in tuberculosis patients, STAT-1, IRF-1, and IRF-9 DNA-binding activity is induced in AM in vivo by treatment with aerosol rIFN-γ (Fig. 3 and 4). Interestingly, in five of five patients coinfected with HIV and M. tuberculosis, including one who received aerosol rIFN-γ as the only change in ongoing chemotherapy, there was a striking decline in the viral load in BAL fluids from segments shown radiographically to be involved (Fig. 5).
STAT-1 DNA-binding activity was undetectable prior to treatment with aerosol rIFN-γ, yet it was expected that at least this transcription factor might have been detected at a low level since endogenous IFN-γ is present at sites of tuberculosis infection (3, 5). We cannot exclude the possibility that the endogenous IFN-γ results in a small amount of activated STAT-1, which begins to decay when BAL is performed, and that BAL cells cannot be collected and extracted expeditiously enough to detect what is left of an initially low level of DNA-binding activity. However, there are at least two alternative explanations for the lack of STAT-1 DNA-binding activity prior to aerosol rIFN-γ treatment. First, the steady-state presence of IFN-γ may result in negative feedback so that normal homeostasis, perhaps through the induction of proteins that are suppressors of cytokine signaling (reviewed in references 1, 27, and 41), limits the amount of activated STAT-1 that is present. Second, immunosuppressive effects of other cytokines, such as IL-10, might limit the response to endogenous IFN-γ. In vitro, IL-10 can limit the activation of STAT-1 in response to IFN-α or IFN-γ (20). The degree of inhibition is smaller at higher doses of the respective IFN. In either case, there might be essentially no activated STAT-1 or it might be present at a level below the limit of detection, which would represent the physiological extent of the response to the physiological presence of IFN-γ.
In comparison to BAL cells obtained after 10 ± 2 days of antituberculosis drug treatment, the induction of STAT-1 and/or IRF-1 and IRF-9 was observed in both uninvolved and involved lobes 4 weeks after aerosol rIFN-γ was added to the treatment regimen. Moreover, induction was greater in BAL cells obtained 1 h after the final aerosol rIFN-γ treatment than in cells obtained 1 day later or was observed only in the cells obtained 1 h after the treatment. These results are consistent with the conclusion from in vitro results that infection alone does not lead to the activation of STAT-1 in human macrophages (Fig. 1) (48) and suggest that the in vivo effects shown here are a response to aerosol rIFN-γ rather than to antituberculosis drug treatment. Altogether, these data demonstrate that the in vitro response of AM to rIFN-γ accords with the paradigm established in studies of secondary cell cultures and tumor cell lines and that, importantly, AM exposed to aerosol rIFN-γ in vivo exhibit activation and induction of transcription factors that mediate response to IFN-γ.
IRF-1 and IRF-9, both of which were induced by aerosol rIFN-γ, allow crossover between the IFN-γ and the IFN-α and IFN-β systems. The importance of the IFN-α/β system for host response to M. tuberculosis is suggested by the good clinical response that has been reported for aerosol IFN-α used as an adjunctive treatment for tuberculosis (15). Giosue and colleagues added aerosol IFN-α to a treatment regimen for 2 months and compared data to those from a control group, observing by computerized tomography scanning that healing accelerated and that there was a more significant decrease in IL-1β, IL-6, and TNF-α in BAL fluids from the IFN-α-treated group (15). The induction of IRF-1 by aerosol IFN-γ is likely to induce a subset of genes that are also regulated by IFN-α through the activation of ISGF-3, since the IRF-1 binding site is contained within the ISGF-3 binding site (36, 38). Such an effect occurs when IRF-1 expression is artificially raised (34), and the lack of IRF-1 limits IFN-α induction of some genes (23, 25, 26). IRF-9 is a subunit of ISGF-3; thus, its induction by IFN-γ directly increases responsiveness to IFN-α. In fact, the observation of just that effect led to the original name for IRF-9, ISGF-3γ (29). These mechanisms may be particularly relevant to tuberculosis, since M. tuberculosis infection of AM in vitro leads to the production of IFN-α/β and the activation of ISGF-3 (48).
While the IFN-α/β system may contribute to host defense against M. tuberculosis (8, 14, 48), the IFN-γ system, including downstream effectors, is clearly critical for resistance to infection by mycobacteria, including M. tuberculosis. Mice with disruptions of genes for IFN-γ (7, 13), IFN-γ receptor (24), IRF-1 (8, 23), or inducible nitric oxide synthetase (8, 30) are more susceptible to mycobacterial infection than wild-type mice. Moreover, normally nonpathogenic mycobacteria can cause disease in humans that have mutations in the IFN-γ receptor or in STAT-1 (10, 11, 22, 33). Our finding that aerosol rIFN-γ upregulates DNA binding by STAT-1 and IRFs in BAL cells in vitro and in tuberculosis patients after 12 aerosol treatments over 1 month suggests that enhanced signaling in cells at the site of disease will lead to increased expression of IFN-γ-responsive genes that participate in the immune response. One such example is the induction of IP-10 by aerosol rIFN-γ in healthy volunteers and in tuberculosis patients (B. Raju, R. Condos, Y. Hoshino, A. Canova, R. Pine, W. Rom, and M. Weiden, unpublished observations). The trends toward decreased production of TNF-α, IL-15, and IFN-γ by BAL cells after 1 month of aerosol rIFN-γ therapy compared to the levels of production after 10 ± 2 days of conventional therapy in the same patients and toward improved clinical symptoms are consistent with augmentation of the immune response. Next, we would recommend a randomized clinical trial comparing directly observed therapy-short course (6 months) to directly observed therapy-short course plus IFN-γ for the first 4 months in a larger number of patients with pulmonary tuberculosis. This may ultimately demonstrate that the duration of conventional therapy can be shortened when conventional therapy is combined with a longer course of aerosol IFN-γ. A propitious clinical outcome would include more rapid conversion of sputum results in advanced cavitary bilateral pulmonary tuberculosis patients, fewer instances of relapse or treatment failure, and greater healing with less tissue destruction.
Acknowledgments
We thank Chris Schindler and David Levy for kind gifts of antibodies.
This work was supported by NIH grants M01 00096, HL 57879, and HL 59832 and by a Doris Duke Clinical Scientist Award to R.C.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Alexander, W. S. 2002. Suppressors of cytokine signalling (SOCS) in the immune system. Nat. Rev. Immunol. 2:410-416. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P. F., and S. A. Barrows. 1993. Tuberculosis in the 1990s. Ann. Intern. Med. 119:400-410. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, P. F., S. D. Mistry, C. L. Cooper, C. Pirmez, T. H. Rea, and R. L. Modlin. 1989. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J. Immunol. 142:1114-1119. [PubMed] [Google Scholar]
- 4.Carson, W. E., M. E. Ross, R. A. Baiocchi, M. J. Marien, N. Boiani, K. Grabstein, and M. A. Caligiuri. 1995. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 96:2578-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condos, R., W. N. Rom, Y. M. Liu, and N. W. Schluger. 1998. Local immune responses correlate with presentation and outcome in tuberculosis. Am. J. Respir. Crit. Care Med. 157:729-735. [DOI] [PubMed] [Google Scholar]
- 6.Condos, R., W. N. Rom, and N. W. Schluger. 1997. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet 349:1513-1515. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in IFNγ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, A. M., J. E. Pearl, J. V. Brooks, S. Ehlers, and I. M. Orme. 2000. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 68:6879-6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 10.Dorman, S. E., and S. M. Holland. 1998. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J. Clin. Investig. 101:2364-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupuis, S., C. Dargemont, C. Fieschi, N. Thomassin, S. Rosenzweig, J. Harris, S. M. Holland, R. D. Schreiber, and J. L. Casanova. 2001. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 293:300-303. [DOI] [PubMed] [Google Scholar]
- 12.Dye, C., S. Scheele, P. Dolin, V. Pathania, M. C. Raviglione, and WHO Global Surveillance and Monitoring Project. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 15.Giosue, S., M. Casarini, L. Alemanno, G. Galluccio, P. Mattia, G. Pedicelli, L. Rebek, A. Bisetti, and F. Ameglio. 1998. Effects of aerosolized interferon-alpha in patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 158:1156-1162. [DOI] [PubMed] [Google Scholar]
- 16.Honda, Y., L. Rogers, K. Nakata, B.-Y. Zhao, R. Pine, Y. Nakai, K. Kurosu, W. N. Rom, and M. Weiden. 1998. Type I interferon induces inhibitory 16-kD CCAAT/enhancer binding protein (C/EBP)β, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J. Exp. Med. 188:1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu, J., S. K. Roy, P. S. Shapiro, S. R. Rodig, S. P. Reddy, L. C. Platanias, R. D. Schreiber, and D. V. Kalvakolanu. 2001. ERK1 and ERK2 activate CCAAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-gamma. J. Biol. Chem. 276:287-297. [DOI] [PubMed] [Google Scholar]
- 18.Hunninghake, G. W., O. Kawanami, V. J. Ferrans, R. C. Young, Jr., W. C. Roberts, and R. G. Crystal. 1981. Characterization of the inflammatory and immune effector cells in the lung parenchyma of patients with interstitial lung disease. Am. Rev. Respir. Dis. 123:407-412. [DOI] [PubMed] [Google Scholar]
- 19.Ihle, J. N. 1996. STATs: signal transducers and activators of transcription. Cell 84:331-334. [DOI] [PubMed] [Google Scholar]
- 20.Ito, S., P. Ansari, M. Sakatsume, H. Dickensheets, N. Vazquez, R. P. Donnelly, A. C. Larner, and D. S. Finbloom. 1999. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 93:1456-1463. [PubMed] [Google Scholar]
- 21.Jaffe, H. A., R. Buhl, A. Mastrangeli, K. J. Holroyd, C. Saltini, D. Czerski, H. S. Jaffe, S. Kramer, S. Sherwin, and R. G. Crystal. 1991. Organ specific cytokine therapy. Local activation of mononuclear phagocytes by delivery of an aerosol of recombinant interferon-gamma to the human lung. J. Clin. Investig. 88:297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jouanguy, E., F. Altare, S. Lamhamedi, P. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956-1961. [DOI] [PubMed] [Google Scholar]
- 23.Kamijo, R., H. Harada, T. Matsuyama, M. Bosland, J. Gerecitano, D. Shapiro, J. Le, S. I. Koh, T. Kimura, S. J. Green, T. W. Mak, T. Taniguchi, and J. Vilcek. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263:1612-1615. [DOI] [PubMed] [Google Scholar]
- 24.Kamijo, R., J. Le, D. Shapiro, E. A. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilcek. 1993. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura, T., Y. Kadokawa, H. Harada, M. Matsumoto, M. Sato, Y. Kashiwazaki, M. Tarutani, R. S.-P. Tan, T. Takasugi, T. Matsuyama, T. W. Mak, S. Noguchi, and T. Taniguchi. 1996. Essential and non-redundant roles of p48 (ISGF3γ) and IRF-1 in both type I and type II interferon responses, as revealed by gene targeting studies. Genes Cells 1:115-124. [DOI] [PubMed] [Google Scholar]
- 26.Kimura, T., K. Nakayama, J. Penninger, M. Kitagawa, H. Harada, T. Matsuyama, N. Tanaka, R. Kamijo, J. Vilcek, T. W. Mak, and T. Taniguchi. 1994. Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science 264:1921-1924. [DOI] [PubMed] [Google Scholar]
- 27.Krebs, D. L., and D. J. Hilton. 2001. SOCS proteins: negative regulators of cytokine signaling. Stem Cells 19:378-387. [DOI] [PubMed] [Google Scholar]
- 28.Law, K. F., J. Jagirdar, M. D. Weiden, M. Bodkin, and W. N. Rom. 1996. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am. J. Respir. Crit. Care Med. 153:1377-1384. [DOI] [PubMed] [Google Scholar]
- 29.Levy, D. E., D. S. Kessler, R. Pine, and J. E. Darnell, Jr. 1989. Cytoplasmic activation of ISGF3, the positive regulator of interferon-α-stimulated transcription, reconstituted in vitro. Genes Dev. 3:1362-1371. [DOI] [PubMed] [Google Scholar]
- 30.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray, C. J., and J. A. Salomon. 1998. Modeling the impact of global tuberculosis control strategies. Proc. Natl. Acad. Sci. USA 95:13881-13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakata, K., W. N. Rom, Y. Honda, R. Condos, S. Kanegasaki, Y. Cao, and M. Weiden. 1997. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am. J. Respir. Crit. Care Med. 155:996-1003. [DOI] [PubMed] [Google Scholar]
- 33.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 34.Pine, R. 1992. Constitutive expression of an ISGF2/IRF1 transgene leads to interferon-independent activation of interferon-inducible genes and resistance to virus infection. J. Virol. 66:4470-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pine, R., A. Canova, and C. Schindler. 1994. Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFN α and IFN γ, and is likely to autoregulate the p91 gene. EMBO J. 13:158-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pine, R., T. Decker, D. S. Kessler, D. E. Levy, and J. E. Darnell, Jr. 1990. Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol. Cell. Biol. 10:2448-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao, Y., S. Prabhakar, E. M. Coccia, M. Weiden, A. Canova, E. Giacomini, and R. Pine. 2002. Host defense responses to infection by Mycobacterium tuberculosis. Induction of IRF-1 and a serine protease inhibitor. J. Biol. Chem. 277:22377-22385. [DOI] [PubMed] [Google Scholar]
- 38.Reich, N. C., and J. E. Darnell, Jr. 1989. Differential binding of interferon-induced factors to an oligonucleotide that mediates transcriptional activation. Nucleic Acids Res. 17:3415-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rom, W. N., N. Schluger, K. Law, R. Condos, Y. Zhang, M. Weiden, T. Harkin, and K. M. Tchou-Wong. 1995. Human host response to Mycobacterium tuberculosis. Schweiz. Med. Wochenschr. 125:2178-2185. [PubMed] [Google Scholar]
- 40.Roy, S. K., S. J. Wachira, X. Weihua, J. Hu, and D. V. Kalvakolanu. 2000. CCAAT/enhancer-binding protein-beta regulates interferon-induced transcription through a novel element. J. Biol. Chem. 275:12626-12632. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto, H., I. Kinjyo, and A. Yoshimura. 2000. The janus kinase inhibitor, Jab/SOCS-1, is an interferon-gamma inducible gene and determines the sensitivity to interferons. Leuk. Lymphoma 38:49-58. [DOI] [PubMed] [Google Scholar]
- 42.Schindler, C., X.-Y. Fu, T. Improta, R. Aebersold, and J. E. Darnell, Jr. 1992. Proteins of transcription factor ISGF-3: one gene encodes the 91- and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc. Natl. Acad. Sci. USA 89:7836-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selwyn, P. A., D. Hartel, V. A. Lewis, E. E. Schoenbaum, S. H. Vermund, R. S. Klein, A. T. Walker, and G. H. Friedland. 1989. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 320:545-550. [DOI] [PubMed] [Google Scholar]
- 44.Sladek, F. M., W. Zhong, E. Lai, and J. E. Darnell, Jr. 1990. Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 4:2353-2365. [DOI] [PubMed] [Google Scholar]
- 45.Ting, L. M., A. C. Kim, A. Cattamanchi, and J. D. Ernst. 1999. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163:3898-3906. [PubMed] [Google Scholar]
- 46.Toossi, Z., K. Nicolacakis, L. Xia, N. A. Ferrari, and E. A. Rich. 1997. Activation of latent HIV-1 by Mycobacterium tuberculosis and its purified protein derivative in alveolar macrophages from HIV-infected individuals in vitro. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 15:325-331. [DOI] [PubMed] [Google Scholar]
- 47.Veals, S. A., C. Schindler, D. Leonard, X.-Y. Fu, R. Aebersold, J. E. Darnell, Jr., and D. E. Levy. 1992. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol. Cell. Biol. 12:3315-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiden, M., N. Tanaka, Y. Qiao, B. Y. Zhao, Y. Honda, K. Nakata, A. Canova, D. E. Levy, W. N. Rom, and R. Pine. 2000. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein β expression. J. Immunol. 165:2028-2039. [DOI] [PubMed] [Google Scholar]
- 49.Weihua, X., V. Kolla, and D. V. Kalvakolanu. 1997. Interferon gamma-induced transcription of the murine ISGF3gamma (p48) gene is mediated by novel factors. Proc. Natl. Acad. Sci. USA 94:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whalen, C., C. R. Horsburgh, D. Hom, C. Lahart, M. Simberkoff, and J. Ellner. 1995. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am. J. Respir. Crit. Care Med. 151:129-135. [DOI] [PubMed] [Google Scholar]