Abstract
Streptococcal toxic shock syndrome (StrepTSS) is an invasive infection characterized by marked coagulopathy, multiple organ failure, and rapid tissue destruction and is strongly associated with M type 1 and 3 group A streptococci (GAS). Initiation of the coagulation cascade with formation of microvascular thrombi contributes to multiple organ failure in human cases of gram-negative bacteremia; however, little is known regarding the mechanism of coagulopathy in StrepTSS. Thus, we investigated the abilities of several strains of M type 1 and 3 GAS isolated from human cases of StrepTSS to stimulate production of tissue factor (TF), the principal initiator of coagulation in vivo. Washed, killed M type 1 and 3 GAS, but not M type 6 GAS, elicited high-level TF-mediated procoagulant activity from both isolated human monocytes and cultured human umbilical vein endothelial cells. M type 1 GAS consistently elicited higher levels of TF from monocytes than did M type 3 GAS. GAS-induced TF synthesis in monocytes did not correlate with production of tumor necrosis factor alpha or interleukin-8. Conversely, M type 3 GAS were consistently more potent than M type 1 GAS in stimulating endothelial cell TF synthesis. These results demonstrate that (i) M type 1 and 3 strains of GAS are potent inducers of TF synthesis, (ii) GAS-induced TF synthesis is not simply an epiphenomenon of cytokine generation, and (iii) induction of TF in endothelial cells and monocytes may be M type specific. In total, these findings suggest that a novel interaction between GAS and host cells contributes to the observed coagulopathy in StrepTSS.
In 1989, Stevens et al. reported a series of patients from the Rocky Mountain West with group A streptococcus (GAS) infections associated with the sudden onset of shock (95%), adult respiratory distress syndrome (55%), renal failure (80%), bacteremia (60%), and deaths (30%) (26). Similar cases emerged worldwide (reviewed in reference 24), prompting an official case definition of streptococcal toxic shock syndrome (StrepTSS) by the Centers for Disease Control Working Group on Streptococcal Infections (36). Despite better clinical recognition of StrepTSS and intense research on streptococcal virulence factors, morbidity is high and mortality remains between 30 and 70% (6). For example, 50% of StrepTSS patients develop necrotizing fasciitis or myonecrosis, often at the site of minor trauma or muscle strain, which rapidly involves an entire extremity (6, 26). Frequently, these deeper infections are not clinically apparent until after shock and renal impairment or adult respiratory distress syndrome are manifest (26). Such patients require emergent amputation or extensive surgical debridement and prolonged hospitalization including ventilator support and hemodialysis (6, 26). In fact, even in the modern era of medicine with its advanced life support technologies, a recent article in the American Journal of Surgery suggested that the best therapy in cases of severe soft tissue infection due to GAS remains a single radical debridement to maximize limb salvage and survival (21).
The mechanisms responsible for this rapid regional destruction of muscle, fascia, and soft tissues have not been studied. It has been our hypothesis that such destruction is due to microvascular thrombosis and endothelial cell dysfunction leading to reduced tissue perfusion, hypoxia, and subsequent regional tissue necrosis. Clinical and experimental observations support this concept. First, both humans and experimental animals with StrepTSS demonstrate a marked coagulopathy characterized by prolonged activated partial thromboplastin time (APTT), reduced fibrinogen, increased fibrin degradation products, and thrombocytopenia. These features are, in fact, part of the case definition of StrepTSS (36). Further, occlusive platelet thrombi and fibrin clots are numerous in capillaries, postcapillary venules, and arterioles of the affected musculature and soft tissues. Taken together, these findings suggest that Streptococcus pyogenes induces systemic activation of the clotting cascade and subverts the physiologic mechanisms controlling hemostasis.
Tissue factor (TF) is the principal initiator of coagulation in vivo (reviewed in reference 31), and its controlled expression is essential for normal hemostasis. This membrane glycoprotein forms a proteolytically active complex with circulating factor VIIa. The TF/VIIa complex then initiates the downstream clotting events of both the intrinsic (via activation of factor IX) and extrinsic (via activation of factor X) coagulation pathways, which culminate in the conversion of prothrombin to thrombin. Thrombin proteolytically cleaves fibrinogen, yielding fibrin monomers that polymerize into a stable clot. In the absence of factor VIIa, TF-initiated clotting does not occur.
Originally, TF activity was thought to be limited to brain, lung, and placenta and to sites in other organs that do not contact flowing blood. Exposure of this extravascular pool of TF following vessel injury, or its induction on endothelial cells following viral infection or exposure to endotoxin or cytokines, was thought to be responsible for both the initiation and the propagation of thrombus formation (18). However, recent studies have demonstrated detectable TF PCA in whole-blood samples from healthy individuals (9, 12). This activity was principally associated with the mononuclear cell fraction (12) and was elevated in patients with sickle cell disease (12). Indeed, increased expression of TF on circulating monocytes has been correlated with a poor prognosis. For example, monocytes isolated from patients with severe meningococcal infection or peritonitis expressed high levels of TF activity (3, 20). Current opinion now suggests that this intravascular pool of TF mediates the pathological propagation of thrombus formation (13), as is observed in septic states associated with disseminated intravascular coagulopathy (DIC).
Because coagulopathy is a defining criterion for StrepTSS, this study investigated the ability of GAS, isolated from patients with invasive infections, to elicit TF production by cultured human umbilical vein endothelial cells and isolated human monocytes.
MATERIALS AND METHODS
Cell culture.
Primary human umbilical vein endothelial cells (HUVEC; BioWhittaker, San Diego, Calif.) were cultured as previously described (7). Briefly, HUVEC from the first through fifth passages were grown in 96-well plates to 70% confluency in growth factor-enriched complete endothelial cell growth medium containing amphotericin-streptomycin (cEGM-2; BioWhittaker) per the manufacturer's recommendations. At this point, cEGM-2 was replaced with basal endothelial cell medium (EBM; BioWhittaker)-4% fetal calf serum and antibiotics and the cell cultures were grown to confluence. At 3 days postconfluency, HUVEC were washed twice with warm EBM containing 2% fetal calf serum without antibiotics (EBM-2). Agonists diluted in EBM-2 were added, and the assays were performed as described below.
Human peripheral blood mononuclear cells were isolated as previously described (10) from healthy volunteers giving informed consent. Briefly, mononuclear cells were isolated by gradient centrifugation over Histopaque (Sigma, St. Louis, Mo.) and residual red blood cells were removed by a single hypotonic lysis. Cells were resuspended at a concentration of 3 × 106/ml in RPMI 1640-penicillin-streptomycin supplemented with 1% pooled human serum (PHS). A total of 3 ml of cells was plated into each well of multiple six-well plates. After 2 h, nonadherent lymphocytes were removed by three washes with prewarmed RPMI 1640 and cell cultures were grown overnight in RPMI 1640-4% PHS plus antibiotics. The next day, this medium was removed and replaced with agonists diluted in RPMI 1640-1% PHS without antibiotics. After 6 h, the procoagulant assay was performed as described below.
Streptococcal strains.
The following strains of GAS were studied: two M type 1 strains (94/059 and 96/004), three M type 3 strains (88/003, 93/040, 94/017), and one M type 6 strain (95/017). Each strain was a blood isolate from a patient clinically diagnosed with StrepTSS. M typing was performed by Dwight Johnson and Edward Kaplan, World Health Organization Reference Laboratory on Streptococci, Minneapolis, Minn. GAS were cultured in sterile polypropylene tubes in 10 ml of Todd-Hewitt broth for 4 to 4.5 h (i.e., log phase) at 37°C in 10% CO2. Organisms were collected by centrifugation, washed twice in ice-cold sterile lipopolysaccharide (LPS)-free normal saline, resuspended in 1.0 ml of saline, and heat killed at 56°C for 1 h as previously described (33). Duplicate platings onto blood agar plates revealed no growth after 24 h. The stock heat-killed organisms were stored at −70°C until use. On the day of the experiment, heat-killed GAS were adjusted to an absorbance at 650 nm of 0.5 in antibiotic-free EBM-2 for HUVEC experiments or RPMI 1640-1% PHS for monocyte studies. An optical density of 0.5 at 650 nm is equivalent to 1 × 108 to 2 × 108 CFU/ml (5). Heat-killed GAS prepared in this way had less than 50 pg of contaminating LPS/ml as determined by a Limulus amoebocyte lysate assay (Associates of Cape Cod, Woods Hole, Mass.). This concentration of GAS, or dilutions thereof, was used to stimulate monocytes or endothelial cells.
TF-mediated PCA assay.
The ability of GAS to stimulate an endothelial cell procoagulant response was investigated using a single-stage clotting (procoagulant activity [PCA]) assay. HUVEC, grown in cultures as described above, were stimulated with 100 μl of heat-killed GAS at an A650 of 0.5, 0.05, or 0.005. Tumor necrosis factor (TNF) (1,000 U/ml), phorbol myristate acetate (5 × 10−6 M), or LPS (5 μg/ml) served as a positive control. After 6 h at 37°C in 5% CO2, the cells were washed twice with prewarmed EBM-2 and each well was inspected for signs of cytotoxicity. All culture media were removed and replaced with 80 μl of lysing solution (20 mM Tris-HCl, 130 mM NaCl, 0.1% bovine serum albumin, pH 7.4), followed by three cycles of freezing and thawing. This latter step is necessary because TF synthesized by activated cells is encrypted and inactive on unperturbed cells (4) but is unmasked by cell disruption (4). Citrated platelet-free human plasma was obtained from healthy volunteers who gave informed consent and who denied taking medication during the previous 10 days. Next, 80 μl of this plasma was added to the reaction mixture and a baseline absorbance reading at 550 nm was taken in a microplate reader. The clotting reaction was initiated by recalcification of the citrated plasma by the addition of 80 μl of 30 mM CaCl2, and absorbance at 550 nm (i.e., clot formation) was monitored every 60 s for 12 to 15 min.
Monocytes were similarly treated, except that cells in six-well plates were lysed with 0.5 ml of lysing solution and duplicate 80-μl samples were transferred to individual wells of a 96-well plate, where citrated plasma and calcium were added and clotting was monitored as described above for HUVEC. In parallel, 100 μl of either the overlying culture medium from monocytes or monocyte lysates was placed in wells of commercial enzyme-linked immunosorbent assay (ELISA) plates for measurement of interleukin-8 (IL-8) and TNF-α (both from R&D Systems, Minneapolis, Minn.) or TF (American Diagnostica, Greenwich, Conn.), respectively, and the assays were performed according to the recommendations of the manufacturers.
In some assays, cells were exposed to agonists for either 1 or 6 h and the clotting assay was performed either in the presence of normal or factor VII-deficient plasma (American Diagnostica) or in the presence of neutralizing anti-human TF antibody (murine immunoglobulin G1, clone TF10-1D10; American Diagnostica) (10 μg/ml final concentration) or an isotype-matched control antibody.
Statistical analyses.
Multiple comparisons between groups were made by one-way analysis of variance with Tukey's test; the level of significance was chosen at P < 0.05.
RESULTS
GAS-induced TF PCA in endothelial cells.
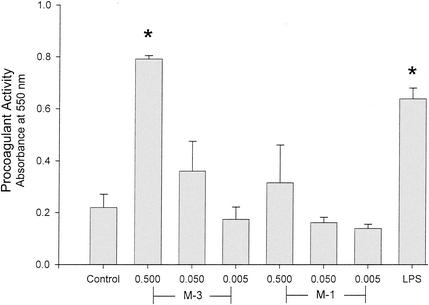
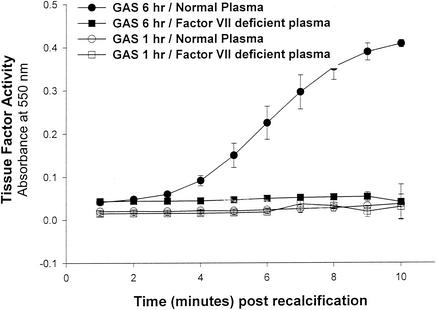
The ability of GAS to stimulate an endothelial cell procoagulant response was determined by a single-stage clotting assay. The three strains of M type 3 GAS tested each stimulated a high level of PCA in endothelial cells comparable to that elicited by LPS (Fig. 1), TNF, or phorbol myristate acetate (data not shown). No clotting was observed in response to the M type 3 strain 88/003 in the absence of factor VII (Fig. 2), suggesting that the PCA was indeed that of TF. Similarly, when HUVEC were exposed to killed M type 3 GAS for only 1 h, no clotting was observed (Fig. 2). Taken together, these findings suggest that the PCA induced by a cell-associated factor of GAS is mediated by the de novo synthesis of TF.
FIG. 1.
M type 3 GAS stimulates endothelial cell PCA. The ability of GAS to elicit PCA in cultured HUVEC was investigated using a single-stage clotting assay. After cultures were grown as described in Materials and Methods, HUVEC were stimulated with 100 μl of heat-killed GAS at an A650 of 0.5, 0.05, or 0.005 for 6 h. Strains tested were M type 1 (strains 96/004 and 94/059) or M type 3 GAS (strains 88/003, 94/017, and 93/040). LPS (5 μg/ml) served as a positive control. After washing and three freeze-thaw cycles, citrated plasma was added followed by CaCl2. PCA (i.e., clot formation) was monitored by absorbance at 550 nm. Data shown are the absorbance values obtained 10 min after recalcification of the plasma. Data (means ± standard deviations [SD]) are representative of four experiments performed in duplicate. *, P < 0.001 by one-way analysis of variance with Tukey's test.
FIG. 2.
Endothelial cell-derived PCA induced by M type 3 GAS is that of TF. PCA in HUVEC was measured by a single-stage clotting assay as described in Materials and Methods. PCA in lysates of HUVEC stimulated for 1 or 6 h with washed, killed M type 3 GAS (A650 = 0.5; strain 88/003) was measured in the presence of either normal plasma or factor VII-deficient plasma. Data are the means of duplicate wells (± SD) minus the mean values of unstimulated control wells.
In contrast to the M type 3 GAS, the two M type 1 strains tested demonstrated a reduced and variable ability to elicit endothelial cell PCA (Fig. 1). Specifically, M type 1 strain 96/004 elicited approximately half as much PCA as the M type 3 strains whereas the M type 1 strain 94/059 was without effect. The single M type 6 strain of GAS tested did not stimulate significant PCA (data not shown). Washed, viable log phase M type 1 GAS demonstrated modest PCA (data not shown) that was inversely related to the concentration of bacteria used, suggesting that cytotoxicity was occurring over the 6-h incubation period. That this was indeed the case was verified by light microscopy.
GAS-induced TF and proinflammatory cytokine production by monocytes.
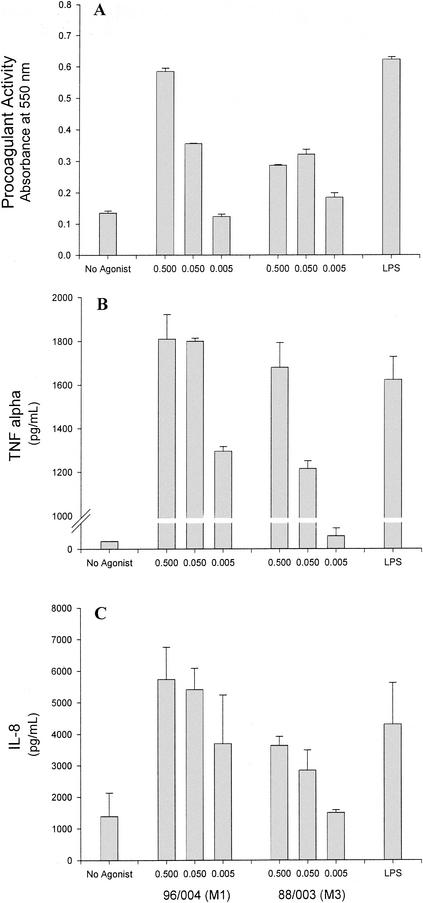
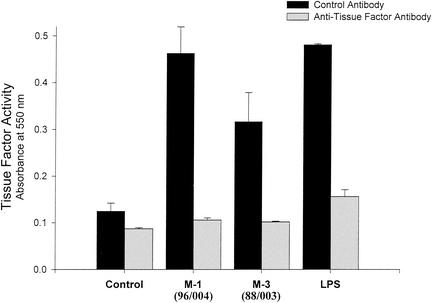
Because monocytes are a significant source of TF in vivo, the ability of GAS to elicit monocyte-derived TF was investigated. M type 1 GAS (strain 96/004) induced a high, dose-dependent level of monocyte-derived PCA comparable to that stimulated by LPS (Fig. 3A). As measured by commercial ELISA, the amounts of TF protein in these lysates in cells stimulated with medium alone, M type 1 GAS, or LPS were 38.2, 88.2, and 135.6 pg/ml, respectively. M type 3 GAS (strain 88/003) induced only modest PCA in monocytes (Fig. 3A). However, both strains stimulated production of high levels of the monokines, TNF-α and IL-8 (Fig. 3B and C, respectively), with the M1 strain again being more potent. Verification that the monocyte PCA elicited by M1 and M3 GAS was indeed TF was obtained by specific neutralization of clotting activity with monoclonal anti-TF antibody (Fig. 4).
FIG.3.
GAS elicit TF PCA and cytokine production in human peripheral blood monocytes. Human peripheral blood monocytes were stimulated for 6 h with either heat-killed M type 1 (strain 96/004) or type 3 (strain 88/003) GAS at an A650 of 0.5, 0.05, or 0.005. LPS (5 μg/ml) served as a positive control. PCA in monocyte lysates (A) was measured in duplicate by a single-stage clotting assay as described in Materials and Methods. Data shown are the absorbance values at 10 min postrecalcification of plasma. The overlying monocyte culture medium was also tested for the presence of TNF-α (B) or IL-8 (C) by commercial ELISAs. These data (means of results from triplicate wells ± SD) are representative of three different experiments utilizing monocytes from different donors; a small but notable donor-dependent variation in TF and cytokine levels was observed (data not shown).
FIG. 4.
Monocyte-derived, GAS-stimulated PCA is that of TF. Monocytes were stimulated with heat-killed GAS of M type 1 (strain 96/004) and 3 (strain 88/003) for 6 h. Monocyte lysates were preincubated for 10 min at 37°C with either monoclonal anti-TF antibody (10 μg/ml) or an isotype-matched control antibody. Normal citrated plasma was added and then recalcified, and clotting was measured. Values depicted are those obtained 12 min after recalcification and represent means of results from triplicate wells ± SD.
DISCUSSION
Necrotizing fasciitis or myonecrosis associated with StrepTSSprogresses rapidly to involve an entire extremity (6, 26). Initiation of the necrotizing process occurs prior to the onset of hypotension, acute respiratory distress syndrome, or multiple organ failure and is characterized by excruciating pain at the site (6, 26). This pain might result from ischemia secondary to vascular compromise related to a bacterium-induced procoagulant state resulting in thrombosis. Indeed, such pain is a prominent feature in other clinical conditions that involve occlusion of the arterial blood supply, such as acute arterial embolism or myocardial infarction. Further, formation of microvascular thrombi with propagation to larger vessels leading to regional vascular occlusion could in fact result in the rapid progression of tissue destruction and multiple organ failure that is characteristic of StrepTSS. Indeed, histologic examination of necrotic tissues obtained from patients with StrepTSS at biopsy or amputation (6, 26) or from experimental animals challenged with GAS (28) reveals clotting in the adjacent microvasculature. In addition, the Centers for Disease Control Working Group on Group A Streptococcal Infections includes coagulopathy (specified as prolongation of the prothrombin time [PT], decreased fibrinogen, increased fibrin degradation products, and thrombocytopenia) as a defining criterion for StrepTSS (36).
Clinical reports and epidemiologic studies have repeatedly shown an association between invasive infections (i.e., bacteremia, necrotizing fasciitis, myonecrosis, and StrepTSS) and GAS strains of M protein types 1, 3, 12, and 28. Among patients having a defined portal of entry, M type 1 and 3 GAS account for over 40% of all isolates (reviewed in reference 24). Further, among the 50% of all StrepTSS patients having no defined portal of entry, virtually all strains of GAS isolated are of M type 1 or 3. Thus, M type 1 and 3 GAS are associated with invasive infections characterized by coagulopathy and rapid tissue destruction.
Although the number of strains tested was small, data presented here clearly demonstrate that M type 1 and 3 strains of GAS possess a cell-associated factor that stimulates production of TF, the principal initiator of blood coagulation in vivo. Indeed, recent studies by Stevens et al. of the responses of experimental animals to GAS infection have demonstrated systemic activation of the coagulation system. Specifically, Stevens et al. have shown that infection with M type 3 GAS was associated with a profound drop in plasma fibrinogen concentration (to 1% of baseline control values), a 50% reduction in platelet count, and marked increases in fibrin degradation products (>640 μg/dl) and in thrombin/anti-thrombin and plasmin/anti-plasmin complexes (25). This coagulopathy was similar to that reported in human cases of StrepTSS (24, 26). Pretreatment of animals with neutralizing monoclonal antibody against TNF improved survival but did not reverse the observed coagulopathy (25), suggesting that the coagulopathy associated with GAS bacteremia was not strictly an epiphenomenon of cytokine generation in the septic state. Data from the present study support this concept in that high levels of TNF-α were induced from monocytes by both M type 1 and M type 3 GAS, yet the M type 3 GAS induced only modest monocyte-derived TF PCA. This implies that an intimate and novel interaction between GAS and host cells is responsible for the observed coagulopathy in StrepTSS.
Results from the present study also suggest that among different M types of GAS, there can be a strain-specific predilection for the TF-producing cells they stimulate. Specifically, M type 3 GAS elicited a high level of TF PCA from endothelial cells, whereas M type 1 strains primarily stimulated monocytes. Such tissue-specific tropism among different GAS strains is not unique to this setting. For instance, it has long been recognized that some strains of M type GAS are primarily skin associated, whereas others are throat strains. Recently, Kalia et al. have demonstrated that this type of tissue tropism and niche separation is associated with specific patterns found in the gene locus for M protein (11). Thus, our findings extend this observation and suggest that over the course of their coevolution, certain M types of GAS have selectively refined their interactions with the human cells regulating hemostasis.
GAS may be unique among pathogenic gram-positive cocci in their ability to stimulate functional responses by endothelial cells that control both inflammation and hemostasis. For instance, Noel et al. (16) have shown that neither viable nor heat-killed Staphylococcus aureus, Enterococcus faecalis, or Streptococcus pneumoniae was able to induce E-selectin expression in cultured endothelial cells. Similarly, Veltrop et al. demonstrated that neither viable nor nonviable Streptococcus sanguis or Staphylococcus epidermidis was able to elicit TF from human vascular endothelial cells (32). In addition, nonviable clinical isolates of S. aureus did not elicit expression of endothelial cell E-selectin or ICAM-1 (27) or TF (32). In contrast, M type 3 GAS are potent inducers of TF synthesis in endothelial cells (Fig. 1). Whether the ability to induce TF synthesis is unique to those M types associated with invasive infection, or whether it is also found among other M types or among M types 1 and 3 associated with noninvasive infections, remains to be determined.
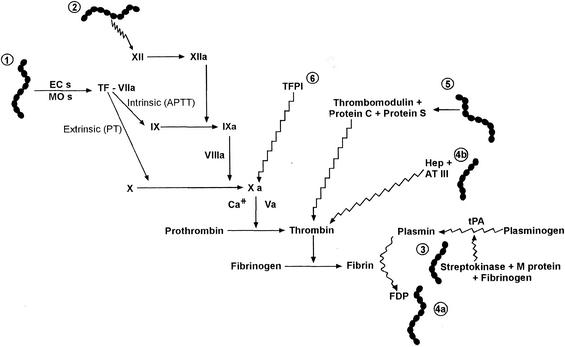
Induction of TF and other known interactions of GAS with the coagulation system is depicted in Fig. 5. In vitro studies have demonstrated that M protein binds fibrinogen, and streptokinase forms a high-affinity complex with plasminogen. Interestingly, as this latter complex then interacts with M protein-bound fibrinogen on the surface of GAS, it acquires potent plasminogen activator activity that is not inhibitable by anti-proteases such as alpha-2-antiplasmin (15, 34). Further, in a murine model of M type 1 GAS necrotizing fasciitis, Sriskandan et al. have recently shown that the APTT was prolonged and was associated with reduced levels of factor XII and prekallikrein (23). Similarly, in a small study of humans with StrepTSS, seven of seven patients had a significantly elevated APTT compared to only one of eight patients having GAS infections not associated with shock and organ failure (22). It can therefore be predicted that patients with severe GAS infections might develop a largely fibrinolytic clinical picture and perhaps a bleeding diathesis. Yet in the murine model mentioned above, the PT was normal and mice actually demonstrated a hypercoagulable state despite prolongation of the APTT (23). Further, in a nonhuman primate model of M type 3 GAS necrotizing fasciitis and myonecrosis, animals had increased circulating levels of fibrin degradation products and thrombin-antithrombin III complexes, indicating systemic activation of the coagulation system (28). Lastly, the marked DIC observed in the lungs (60%), adrenals (100%), and kidneys (80%) of primates with streptococcal bacteremia (25) and in tissues from humans with StrepTSS (our unpublished data) also suggests that the hemostatic balance is shifted toward a pathological TF-mediated procoagulant state in severe GAS infections (Fig. 5).
FIG. 5.
Schematic of known interactions of GAS with the human coagulation system. Straight lines indicate pathways that contribute to coagulation; wavy lines indicate pathways that inhibit coagulation or contribute to fibrinolysis. (Interaction 1) M type 1 and 3 GAS stimulate TF production from endothelial cells (ECs) and monocytes (MOs) (this study). (Interaction 2) In experimental GAS necrotizing fasciitis induced by an M type 1 strain of GAS (23) and in humans with StrepTSS (22), Factor XII was decreased and the APTT was prolonged; however, the PT was normal and experimental animals displayed a hypercoagulable state (23). (Interaction 3) Streptokinase binds plasminogen and GAS M protein binds fibrinogen. This quaternary complex has potent plasminogen activator activity (15, 34). (Interaction 4) In a nonhuman primate model of M type 3 GAS necrotizing fasciitis and myonecrosis, animals had increased circulating levels of fibrin degradation products (FDP) (interaction 4a) and thrombin-antithrombin III (AT III) complexes (interaction 4b), indicating systemic activation of the coagulation system (28). (Interaction 5) Some strains of GAS, but notably not M type 1 or 3, bind protein S in plasma (30). (Interaction 6) Administration of TFPI in experimental animals with gram-negative bacteremia or in humans with sepsis has proven benefits; however, the efficacy of TFPI in StrepTSS remains to be determined.
For a hypercoagulable state to progress to clinical DIC in invasive S. pyogenes infections, the opposing anticoagulant systems (e.g., TF pathway inhibitor [TFPI] and thrombomodulin/protein C/protein S complex) must also be functionally downregulated (Fig. 5). Interestingly, certain strains of GAS bind protein S in its plasma and endothelial cell-bound forms (30). In addition, some S. pyogenes strains bind C4BP (29), a serum protein that complexes with free protein S. In terms of the coagulation system, we suggest that streptococcal binding of protein S effectively removes this molecule from participation in the anticoagulant system and thereby promotes coagulation. However, the role of protein S binding in the pathogenesis of invasive streptococcal disease remains unclear, since serotypes of GAS that are commonly associated with invasive infections (i.e., M type 1 or 3) do not bind protein S (14) or C4BP (29).
Under disease conditions associated with DIC, a massive production and continuous exposure of excess TF is thought to exhaust the available TFPI (reviewed in reference 19). This concept formed the basis for several efficacy studies of TFPI with both humans and experimental animals with gram-negative sepsis. Administration of TFPI limited the development of acute lung and renal injury (35), DIC, and mortality (8) in baboons with Escherichia coli bacteremia and reduced mortality in mice with polymicrobial intra-abdominal infection (17). Similarly, in recent studies in humans, administration of TFPI to critically ill septic patients showed a trend toward reduction in 28-day mortality (1, 2). The efficacy of this therapeutic strategy in the treatment of StrepTSS remains to be tested.
In summary, the data presented here suggest that M type 1 and 3 strains of GAS possess unique cell-associated components that elicit important functional responses in cells of the coagulation-hemostasis system. In vivo, these responses may contribute to intravascular thrombosis and leukostasis, multiple organ failure, and rapid destruction of tissue characteristic of StrepTSS. Understanding the specific mechanisms by which streptococcal virulence factors interact with human cells might offer new insights into the pathogenesis of StrepTSS while providing novel therapeutic targets to limit the severity of this invasive disease.
Acknowledgments
This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Administration, and by a COBRE grant from the National Institutes of Health (NCRR P20 RR15587).
We thank Ronald Bach for helpful discussions and review of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Abraham, E. 2000. Tissue factor inhibition and clinical trial results of tissue factor pathway inhibitor in sepsis. Crit. Care Med. 28:S31-S33. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, E., K. Reinhart, P. Svoboda, A. Seibert, D. Olthoff, A. Dal Nogare, R. Postier, G. Hempelmann, T. Butler, E. Martin, C. Zwingelstein, S. Percell, V. Shu, A. Leighton, and A. A. Creasey. 2001. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo-controlled single-blind, dose escalation study. Crit. Care Med. 29:2081-2089. [DOI] [PubMed] [Google Scholar]
- 3.Almdahl, S. M., J. H. Brox, and B. Osterud. 1987. Mononuclear phagocyte thromboplastin and endotoxin in patients with secondary bacterial peritonitis. Scand. J. Gastroenterol. 22:914-918. [DOI] [PubMed] [Google Scholar]
- 4.Bach, R. R. 1998. Mechanism of tissue factor activation on cells. Blood Coagul. Fibrinolysis 9:S37-S45. [PubMed] [Google Scholar]
- 5.Barry A. 1991. Procedures and theoretical considerations for testing antimicrobial agents in agar media, p. 1-16. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 6.Bisno, A. L., and D. L. Stevens. 1996. Streptococcal infections in skin and soft tissues. N. Engl. J. Med. 334:240-245. [DOI] [PubMed] [Google Scholar]
- 7.Bryant, A. E., and D. L. Stevens. 1996. Phospholipase C and perfringolysin O from Clostridium perfringens upregulate endothelial cell-leukocyte adherence molecule 1 and intercellular leukocyte adherence molecule 1 expression and induce interleukin-8 synthesis in cultured human umbilical vein endothelial cells. Infect. Immun. 64:358-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creasey, A. A., A. C. K. Chang, L. Feigen, T. C. Wun, F. B. Taylor, and L. B. Hinshaw. 1993. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J. Clin. Investig. 91:2850-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giesen, P. L. A., U. Rauch, B. Bohrmann, D. Kling, M. Roque, J. T. Fallon, J. J. Badimon, J. Himber, M. A. Riederer, and Y. Nemerson. 1999. Blood-borne tissue factor: another view of thrombosis. Proc. Natl. Acad. Sci. USA 96:2311-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackett, S. P., and D. L. Stevens. 1992. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J. Infect. Dis. 165:879-885. [DOI] [PubMed] [Google Scholar]
- 11.Kalia, A., B. G. Spratt, M. C. Enright, and D. E. Bessen. 2002. Influence of recombination and niche separation on the population genetic structure of the pathogen Streptococcus pyogenes. Infect. Immun. 70:1971-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Key, N. S., A. Slungaard, L. Dandelet, S. C. Nelson, C. Moertel, L. A. Styles, F. A. Kuypers, and R. R. Bach. 1998. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood 91:4216-4223. [PubMed] [Google Scholar]
- 13.Konigsberg, W., D. Kirchhofer, M. A. Riederer, and Y. Nemerson. 2001. The TF:VIIa complex: clinical significance, structure-function relationships and its role in signaling and metastasis. Thromb. Haemostasis 86:757-771. [PubMed] [Google Scholar]
- 14.Liang, O. D., K. T. Preissner, and G. S. Chhatwal. 1997. The hemopexin-type repeats of human vitronectin are recognized by Streptococcus pyogenes. Biochem. Biophys. Res. Commun. 234:445-449. [DOI] [PubMed] [Google Scholar]
- 15.Lottenberg, R., C. C. Broder, and M. D. P. Boyle. 1987. Identification of a specific receptor for plasmin on a group A streptococcus. Infect. Immun. 55:1914-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel, R. F., T. T. Sato, C. Mendez, M. C. Johnson, and T. H. Pohlman. 1995. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria requires soluble CD14. Infect. Immun. 63:4046-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opal, S. M., J. E. Palardy, N. A. Parejo, and A. A. Creasey. 2001. The activity of tissue factor pathway inhibitor in experimental models of superantigen-induced shock and polymicrobial intra-abdominal sepsis. Crit. Care Med. 29:205-207. [DOI] [PubMed] [Google Scholar]
- 18.Osterud, B., M. S. Bajaj, and S. P. Bajaj. 1995. Sites of tissue factor pathway inhibitor (TFPI) and tissue factor expression under physiologic and pathologic conditions. Thromb. Haemostasis 73:873-875. [PubMed] [Google Scholar]
- 19.Osterud, B., and E. Bjorklid. 2001. The tissue factor pathway in disseminated intravascular coagulation. Semin. Thromb. Hemost. 27:605-617. [DOI] [PubMed] [Google Scholar]
- 20.Osterud, B., and T. Flaegstad. 1983. Increased thromboplastin activity in monocytes of patients with meningococcal infection: related to an unfavourable prognosis. Thromb. Haemostasis 49:5-7. [PubMed] [Google Scholar]
- 21.Schurr, M., S. Engelhardt, and R. Helgerson. 1998. Limb salvage for streptococcal gangrene of the extremity. Am. J. Surg. 175:213-217. [DOI] [PubMed] [Google Scholar]
- 22.Sriskandan, S., and J. Cohen. 2000. Kallikrein-kinin system activation in streptococcal toxic shock syndrome. Clin. Infect. Dis. 30:961-962. [DOI] [PubMed] [Google Scholar]
- 23.Sriskandan, S., G. Kemball-Cook, D. Moyes, J. Canvin, E. Tuddenham, and J. Cohen. 2000. Contact activation in shock caused by invasive group A Streptococcus pyogenes. Crit. Care Med. 28:3684-3691. [DOI] [PubMed] [Google Scholar]
- 24.Stevens, D. L. 1992. Invasive group A streptococcus infections. Clin. Infect. Dis. 14:2-13. [DOI] [PubMed] [Google Scholar]
- 25.Stevens, D. L., A. E. Bryant, S. P. Hackett, A. Chang, G. Peer, S. Kosanke, T. Emerson, and L. Hinshaw. 1996. Group A streptococcal bacteremia: the role of tumor necrosis factor in shock and organ failure. J. Infect. Dis. 173:619-626. [DOI] [PubMed] [Google Scholar]
- 26.Stevens, D. L., M. H. Tanner, J. Winship, R. Swarts, K. M. Reis, P. M. Schlievert, and E. Kaplan. 1989. Reappearance of scarlet fever toxin A among streptococci in the Rocky Mountain West: severe group A streptococcal infections associated with a toxic shock-like syndrome. N. Engl. J. Med. 321:1-7. [DOI] [PubMed] [Google Scholar]
- 27.Strindhall, J., P.-E. Lindgren, S. Lofgren, and E. Kihlstrom. 2002. Variations among clinical isolates of Staphylococcus aureus to induce expression of E-selectin and ICAM-1 in human endothelial cells. FEMS Immunol. Med. Microbiol. 32:227-235. [DOI] [PubMed] [Google Scholar]
- 28.Taylor, F. B., Jr., A. E. Bryant, K. E. Blick, E. Hack, P. M. Jansen, S. D. Kosanke, and D. L. Stevens. 1999. Staging of the baboon response to group A streptococcus administered intramuscularly: a descriptive study of the clinical symptoms and clinical chemical response. Clin. Infect. Dis. 29:167-177. [DOI] [PubMed] [Google Scholar]
- 29.Thern, A., L. Stenberg, B. Dahlback, and G. Lindahl. 1995. Ig-binding surface proteins of Streptococcus pyogenes also bind human C4b-binding protein (C4BP), a regulatory component of the complement system. J. Immunol. 154:375-386. [PubMed] [Google Scholar]
- 30.Valentin-Weigand, P., J. Grulich-Henn, G. S. Chhatwal, G. Müller-Berghaus, H. Blobel, and K. T. Preissner. 1988. Mediation of adherence of streptococci to human endothelial cells by complement S protein (vitronectin). Infect. Immun. 56:2851-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Gorp, E. C. M., C. Suharti, H. ten Cate, W. M. V. Dolmans, J. W. M. van der Meer, J. W. ten Cate, and D. P. M. Brandjes. 1999. Review: infectious diseases and coagulation disorders. J. Infect. Dis. 180:176-186. [DOI] [PubMed] [Google Scholar]
- 32.Veltrop, M. H. A. M., H. Beehuizen, and J. Thompson. 1999. Bacterial species- and strain-dependent induction of tissue factor in human vascular endothelial cells. Infect. Immun. 67:6130-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villaseñor-Sierra, A., A. E. Bryant, and D. L. Stevens. 1999. Type-specific opsonophagocytosis of group A streptococcus using a rapid chemiluminescence assay. J. Infect. Dis. 179:1293-1296. [DOI] [PubMed] [Google Scholar]
- 34.Wang, H., R. Lottenberg, and M. Boyle. 1994. Analysis of plasmin(ogen) acquisition by clinical isolates of Group A streptococci incubated in human plasma. J. Infect. Dis. 169:143-149. [DOI] [PubMed] [Google Scholar]
- 35.Welty-Wolf, K. E., M. S. Carraway, D. L. Miller, T. L. Ortel, M. Ezban, A. J. Ghio, S. Idell, and C. A. Piantadosi. 2001. Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am. J. Respir. Crit. Care Med. 164:1988-1996. [DOI] [PubMed] [Google Scholar]
- 36.The Working Group on Severe Streptococcal Infections. 1993. Defining the group A streptococcal toxic shock syndrome: rationale and consensus definition. JAMA 269:390-391. [PubMed] [Google Scholar]