Abstract
Enteropathogenic Escherichia coli (EPEC) strains are a common cause of persistent diarrhea among infants, primarily in developing countries. The pathogenicity of EPEC is associated with the expression and secretion of bacterial proteins encoded by the chromosomal locus of enterocyte effacement (LEE). The LEE-encoded type III-secreted proteins EspA, EspB, and EspD are part of a molecular syringe, which is used by EPEC to translocate effector proteins directly into the cytoplasm of host cells. The type III-secreted translocated intimin receptor (Tir) protein is thought to be delivered by an Esp-dependent mechanism into the host cell, and this is followed by insertion into the host plasma membrane, where the protein serves as the receptor for intimin, an afimbrial bacterial adhesin. Type III secretion is subject to environmental regulation, and secretion can be induced in vitro by growing bacteria in cell culture medium. In this study we found that Ca2+ is involved in the regulation of type III secretion both in classical locally adherent EPEC and in atypical diffusely adherent EPEC. Interestingly, we observed contrasting secretion of Esp proteins and Tir in response to Ca2+. While the secretion of Tir is clearly enhanced and the protein is integrated into HeLa membranes under calcium chelation conditions, Esp secretion is strongly reduced under these conditions. These data suggest that under Ca2+-depleted conditions Tir might be secreted into the medium and integrated into host membranes by an Esp-independent mechanism, without the need for a functional type III translocation machinery.
Enteropathogenic Escherichia coli (EPEC) strains are a major cause of persistent bacterial diarrheal disease, especially in young children (10, 32, 49). A characteristic feature of EPEC is the induction of attaching and effacing (A/E) lesions in infected epithelial cells. This process is associated with localized degeneration of the brush border microvilli, followed by intimate bacterial adherence to a membrane protrusion (pedestal) generated by the underlying newly polymerized actin (reviewed in references 17 and 24). EPEC strains harbor a unique 35-kb pathogenicity island, the locus of enterocyte effacement (LEE), which contains all the genes necessary for the formation of A/E lesions (33). Homologues of the LEE have been identified in several other types of pathogenic and A/E-inducing E. coli, including enterohemorrhagic E. coli (EHEC) and atypical diffusely adhering E. coli (DA-EPEC) (5). At least 20 genes of the EPEC LEE have been found to encode a type III secretion system (TTSS) (23). This specialized protein secretion system is designed to deliver effector proteins, which are associated with pathogenicity, across the bacterial membrane into the host cell (for reviews, see references 21 and 43). The LEE-encoded type III-secreted proteins (Esp) of EPEC, EspA, EspB, and EspD, are required for induction of the characteristic A/E phenotype (15, 29, 30). Both EspB and EspD are inserted into the target cell membrane, thereby forming a pore (6, 17, 22, 50, 53). EspA is assembled into a unique filamentous structure that is also required for protein translocation because it provides a conduit connecting the EPEC TTSS and the translocation pore with the host cell membrane (6). The contact-dependent hemolytic activity of EPEC can be explained by the ability of the organism to form pores in the host cell membrane. The LEE-encoded and type III-secreted translocated intimin receptor (Tir) (72 to 78 kDa) is inserted into the plasma membrane of the host cell, where it acts as a receptor for intimin (7, 27). The binding of intimin to Tir results in a profound rearrangement of the actin cytoskeleton, which leads to the characteristic pedestal formation beneath adherent bacteria. Infection experiments performed with HeLa cells and EPEC have demonstrated that the delivery of Tir into the host cell is facilitated by EspA and EspB (27).
DA-EPEC strains, like 3431 and B6, secrete proteins that are homologues of the EPEC type III-secreted EspA, EspB, EspD, and Tir proteins. However, in contrast to classical EPEC strains, DA-EPEC does not express bundle-forming pili, which, together with intimin, are the major attachment factors of classical EPEC strains. Recently, we showed that DA-EPEC strains 3431 and B6 are also able to induce Esp-dependent hemolysis, but in contrast to classical EPEC cell-cell contact is not required (22).
Expression and secretion of virulence proteins of pathogenic gram-negative bacteria are tightly regulated (34). This permits the coordination of protein expression and secretion in response to changes in environmental conditions or to different phases of infection. Ca2+ is a well-known regulator of various cellular processes and has also been found to be involved in regulation of the expression of virulence factors during bacterial pathogenesis (e.g., in Yop secretion by Yersinia [48]). The secretion of Esp proteins by EPEC and DA-EPEC is influenced by environmental factors like temperature, culture medium, pH, iron concentration, and osmolarity (3, 26). Furthermore, it has been shown that Ca2+ is needed for efficient Esp secretion by EPEC strain E2348/69 (26).
In this study, we examined the effect of Ca2+on type III secretion and on hemolytic activity of DA-EPEC. We found that Ca2+ has a differential effect on TTSS-mediated secretion of effector proteins. Our data strongly suggest that there is an alternative mechanism of Tir delivery and integration into host cells that is triggered under low-Ca2+ conditions and is Esp independent.
(Part of this research was conducted by T. Ide and S. Michgehl in partial fulfillment of the requirements for Ph.D. degrees from the Westfälische Wilhelms-Universität Münster, Münster, Germany.)
MATERIALS AND METHODS
Bacterial strains, tissue culture cell lines, and culture conditions.
DA-EPEC strains were isolated from diarrhea patients in Germany (strain B6, provided by G. Peters, Münster, Germany) and Brazil (strain 3431, provided by L. R. Trabulsi, Saõ Paulo, Brazil). The EPEC prototype strain E2348/69 was obtained from J. B. Kaper (Baltimore, Md.). The ΔespB EPEC strain UMD864, the ΔespD EPEC strain UMD870, and the EPEC type III secretion mutant cfm 14-2-1 were kindly provided by M. Donnenberg (Baltimore, Md.). All strains were routinely stored at −70°C in standard I medium (Merck, Darmstadt, Germany) containing 15% glycerol. HeLa cells (ATCC CCL 2; human cervical epitheloid carcinoma) were routinely grown at 37°C in a 10% CO2 atmosphere in Dulbecco's minimal essential medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum, 1 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml).
Isolation and SDS-polyacrylamide gel analysis of secreted proteins.
Bacteria were grown without agitation (static cultures) in DMEM or DMEM containing 5 mM EGTA for 3 h at 37°C in the presence of 10% CO2. The bacteria were pelleted by repeated centrifugation (once at 3,000 × g and 4°C for 15 min and twice at 17,000 × g and 4°C for 15 min). To precipitate proteins released into the supernatant, trichloroacetic acid (TCA) was added to a final concentration of 10% (wt/vol), and the mixture was incubated on ice for at least 1 h. Precipitated proteins were collected by centrifugation (17,000 × g, 4°C, 15 min), washed once with ice-cold 90% (vol/vol) acetone, resuspended in loading buffer (10% glycerol, 1.5% sodium dodecyl sulfate [SDS], 4% 2-mercaptoethanol, 30 mM Tris-HCl; pH 6.8), and boiled for 15 min. After separation by SDS-12.5% polyacrylamide gel electrophoresis (PAGE), proteins were stained with Coomassie brilliant blue.
Western blot analysis of type III-secreted proteins.
Precipitated bacterial supernatant or bacterial whole-cell extracts were subjected to SDS-12.5% PAGE and transferred to nitrocellulose membranes for immunoblot analysis performed with polyclonal mouse antisera against EspD and EspB as described previously (4). A monoclonal anti-Tir antibody (B51) directed against Tir of (Shiga-toxin-producing E. coli (STEC) strain 413/89-1 (7) (kindly provided by F. Ebel, Munich, Germany) specifically recognizes Tir of EPEC strain E2348/69 and DA-EPEC strain 3431 and was used for immunodetection.
Quantification of hemolysis of SRBC by bacteria and bacterial supernatants.
The hemolysis assay was performed essentially as described previously (4, 20, 39). Briefly, sheep red blood cells (SRBC) were mixed 1:1 with bacteria that were grown for 3 h in DMEM with or without 5 mM EGTA without agitation or with an equal volume of bacterial supernatant and incubated for 1 h at 37°C in the presence of 10% CO2; 10 mM CaCl2 was added to the bacterial culture or the supernatant immediately before it was mixed with SRBC. The lysis of SRBC was monitored by monitoring the release of hemoglobin photometrically at 450 nm. The background (B) was defined as the optical density at 450 nm of SRBC incubated with DMEM. For complete hemolysis (T), erythrocytes were lysed with 0.2% (wt/vol) Saponin (Sigma, Taufkirchen, Germany). The percentage of total hemolysis (P) was calculated by using the following equation: P = [(X − B)/(T − B)] × 100, where X is the optical density of the sample. The data below are the mean values for three independent assays performed in duplicate.
RNA isolation and RT-PCR.
Total bacterial RNA from 1.5-ml samples obtained from DMEM static cultures (with or without 5 mM EGTA) was isolated by using Trireagent (Sigma, Taüfkirchen, Germany) according to the manufacturer's instructions. RNA preparations were treated with DNAfree (Ambion, Huntindon, United Kingdom). cDNA was synthesized with Moloney murine leukemia virus reverse transcriptase (Promega, Mannheim, Germany). The oligonucleotide primer pairs used for the reverse transcription (RT)-PCR analysis are listed in Table 1. 16S rRNA-specific oligonucleotides were used to monitor RNA quality and to equilibrate the loading concentrations. PCRs with the RT products were performed by using standard procedures and Taq DNA polymerase.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| espB3431(+) | TCCCCCGGGATGAATACTATTGATAATAAT |
| espB3431(−) | TCCCCCGGGTTACCCGGCTAAGCGACCCGA |
| espD3431(+) | AATCTGTTCACGCTAGGCGGATCCGCGATGCTTAATGTAAATAACGATATCC |
| espD3431(−) | TAAACCAATTCCCCCGGGGGATTAAATTCGACCACTAAC |
| 16S rRNA(+) | CCGAATTCGTCGACAACAGAGTTTGATCCTGGCTCAG |
| 16S rRNA(−) | ACCGCGGCTGCTGGCACGGAGTTAGCCGGT |
| HisTir 2348(+) | AAGGATCCATGCCTATTGGTAACCTTG |
| His Tir 2348(−) | CCGCTCGAGAACGAAACGTACTGGTCCCGG |
| His Tir 3431(+) | GGAATTCCATATGCCTATTGGTAACCTTGG |
Plasmid construction and protein purification.
The tir genes were amplified by using as templates chromosomal DNA from EPEC strain E2348/69 and DA-EPEC strain 3431. Oligonucleotides HisTir 2348(+) and HisTir 2348(−) were designed to introduce BamHI and XhoI restriction sites for tir from EPEC strain E2348/69. HisTir 3431(+) and HisTir 2348(−) were used to introduce NdeI and XhoI restriction sites for tir from DA-EPEC strain 3431, respectively (Table 1). The PCR-amplified fragments were digested with the appropriate restriction enzymes and cloned into pET24b (Novagen, Schwalbach, Germany), a vector containing a His epitope tag, resulting in C-terminally tagged proteins.
For production of His-tagged proteins, overnight cultures of strain DH5α harboring plasmids pTir2348His and pTir3431His were diluted 1:100 into 150 ml of Luria-Bertani medium with 150 μg of ampicillin per ml and grown to an optical density at 600 nm of 0.6. Then 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added, and the cultures were incubated for an additional 3 h at 37°C. Bacteria were harvested by centrifugation. His-tagged proteins were purified with the QIAGEN purification system. Protein concentrations were assessed by Coomassie blue staining of SDS-PAGE gels by using standard proteins.
Fractionation of HeLa cells and protein extraction.
Bacteria were grown without agitation in DMEM containing 5 mM EGTA for 3 h in the presence of 10% CO2, and 15 to 20 ml of filter-sterilized bacterial supernatant was added to HeLa monolayers grown in tissue dishes. After 2 h of incubation, the cells were washed three times with cold phosphate-buffered saline. The cells were resuspended in 1 ml of sonication buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 30% glycerol, 0.4 mM NaVO3, 2 mM NaF) supplemented with 8% (vol/vol) 25× Complete EDTA-free protease inhibitor mixture (Roche Biochemicals, Mannheim, Germany) and sonicated four times for 1 s to rupture the HeLa cell membranes. The sonicate was centrifuged at 4°C for 30 min at 108,000 × g. The resulting supernatant contained the soluble cytosolic proteins (cytosolic fraction). The pellet was resuspended in 1 ml of sonication buffer supplemented with 1% Triton X-100, incubated at 4°C for 30 min, and centrifuged at 4°C for 30 min at 108,000 × g. The cytosolic fraction and the supernatant containing the Triton X-100-soluble membrane proteins (membrane fraction) were mixed with loading buffer (10% glycerol, 1.5% SDS, 4% 2-mercaptoethanol, 30 mM Tris-HCl [pH 6.8]), separated by SDS-PAGE, and analyzed by Western blotting. Membrane fractions and cytosolic fractions of HeLa cells incubated with EPEC strain E2348/69 supernatant were precipitated with TCA prior to separation by SDS-PAGE.
RESULTS
Esp secretion by DA-EPEC is Ca2+ dependent.
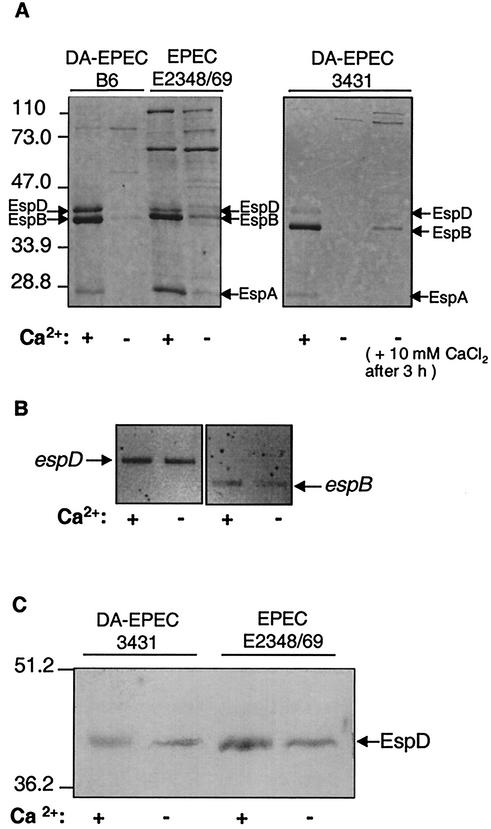
It has been shown previously that the secretion of Esp proteins by the TTSS in EPEC strain E2348/69 is dependent on Ca2+ (26). To further elucidate the influence of Ca2+ on Esp secretion in the prototype EPEC strain and to investigate the effect of Ca2+ on the TTSS secretion of effector proteins in LEE-positive diffusely adhering E. coli strains, we analyzed supernatants from DA-EPEC and EPEC strains grown in Ca2+-depleted or Ca2+-supplemented DMEM as described in Materials and Methods. As shown by SDS-PAGE, DA-EPEC strains 3431 and B6, as well as EPEC strain E2348/69, displayed the characteristic pattern of type III-secreted proteins, accentuated for EspD, EspB, and EspA, when they were grown in DMEM (Fig. 1A). However, in Ca2+-depleted medium the Esp secretion by both DA-EPEC strains and by EPEC strain E2348/69 was strongly reduced. Bacterial growth was not affected. Interestingly, EGTA reduced Esp secretion more efficiently in DA-EPEC than in EPEC strain E2348/69 (Fig. 1A). As shown for DA-EPEC strain 3431, secretion of Esp could be rapidly restored by addition of 10 mM CaCl2 for 1 h (Fig. 1A).
FIG. 1.
Ca2+ dependence of Esp expression and secretion. Bacteria were grown for 3 h at 37°C as static cultures in DMEM containing 1.8 mM CaCl2 (+) or with Ca2+ depleted (−) by addition of 5 mM EGTA. Bacteria were removed from each supernatant by centrifugation. Precipitated proteins of the supernatant (A) or whole-cell extracts (C) were separated by SDS-12.5% PAGE and visualized by Coomassie brilliant blue staining or Western blot analysis with anti-EspD (C). Volumes corresponding to equal amounts of bacteria were loaded in all lanes. (B) RT-PCR analysis of espB and espD transcription. RNA was extracted from DA-EPEC strain 3431 and subjected to RT-PCR by using oligonucleotides specific for espB and espD amplification. To ensure that the same amount of RNA was used in each RT-PCR, RT-PCRs with oligonucleotides specific for 16S rRNA were included as controls (data not shown).
Ca2+ influences secretion and not transcription of EspB and EspD.
To investigate whether the reduction in Esp secretion by DA-EPEC under Ca2+-depleted conditions might be due to reduced type III secretion or, alternatively, might be a result of reduced Esp expression, we carried out RT-PCR with espB- or espD-specific primers and mRNA of DA-EPEC strain 3431 grown in the presence or absence of Ca2+. To ensure that the amount of PCR product reflects the amount of cDNA in the reaction, controls were carried out with larger amounts of cDNA. RT-PCR with 16S rRNA-specific oligonucleotides was included to ensure that there were equal amounts of RNA in all RT-PCRs (data not shown). As shown for espB and espD, the Ca2+ concentration had no effect on Esp transcription (Fig. 1B). Western blot analysis of whole-cell pellets of DA-EPEC strain 3431 and EPEC strain E2348/69 demonstrated that calcium chelation did not affect the intracellular level of EspD (Fig. 1C). This indicates that Ca2+ influences the secretion of Esp proteins by the TTSS but not their expression.
EPEC and DA-EPEC hemolytic activity is influenced by Ca2+.
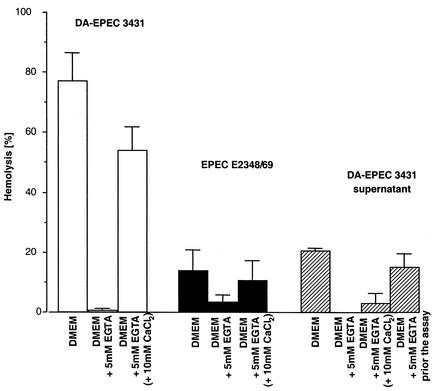
Based on the Esp-mediated hemolytic activity of EPEC and DA-EPEC, we expected that the effect of Ca2+ on type III secretion would also affect the ability of the bacteria to hemolyze red blood cells. Therefore, we infected SRBC with DA-EPEC strain 3431 or EPEC strain E2348/69 grown in DMEM with or without 5 mM EGTA as described in Materials and Methods. The DA-EPEC strain showed the expected hemolytic activity (about 80%) when it was grown in DMEM (Fig. 2). In contrast, infection of SRBC with DA-EPEC grown in DMEM without Ca2+ did not induce hemolysis. All assays were carried out without a centrifugation step, which has often been used to enhance the contact between blood cells and bacteria. As hemolysis by EPEC strain E2348/69 is contact dependent (52), the highest level of hemolytic activity of this strain was only 20%. However, when the organism was grown without Ca2+, the hemolytic activity of EPEC strain E2348/69 was reduced even further. Addition of CaCl2 prior to infection restored the hemolytic activity, which is in accordance with the recovery of Esp secretion after the reconstitution of Ca2+ levels (Fig. 2).
FIG. 2.
Ca2+ dependence of hemolytic activity. Hemolysis assays were performed as described in Materials and Methods by using SRBC and bacteria at a ratio of 1:1 or supernatant of a bacterial culture grown with or without Ca2+. The release of hemoglobin was monitored by measuring the optical density at 450 nm.
The Esp proteins in the supernatants of DA-EPEC strains 3431 and B6 are sufficient for pore formation (22). However, the supernatant derived from DA-EPEC strain 3431 grown without Ca2+ showed no hemolytic activity (Fig. 2). If Ca2+ was added, hemolytic activity that was 15% of the hemolytic activity of supernatant derived from bacteria grown in the presence of Ca2+ was restored. Most likely, this activity can be attributed to the presence of some residual bacteria in the supernatant. For hemolysis assays, bacteria cannot be removed from the supernatants by filter sterilization, as Esp proteins bind to filter membranes. Furthermore, addition of EGTA to the Esp-containing supernatant immediately before incubation with the erythrocytes had only a marginal effect on hemolysis (Fig. 2). Taken together, these results indicate that Ca2+ influences the bacterial hemolytic activity by inhibiting Esp secretion and not by inhibiting Esp activity.
Ca2+ dependence of Tir secretion.
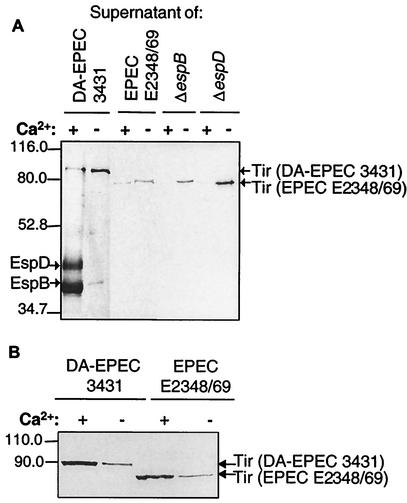
As demonstrated in Fig. 1A, Ca2+ is needed for optimal secretion of EspA, EspD, and EspB. Interestingly, depletion of Ca2+ in the culture media of DA-EPEC and EPEC strains increased the secretion of proteins with apparent molecular masses of 78 and 90 kDa, respectively. SDS-PAGE of TCA-precipitated supernatants from DA-EPEC strain 3431 and EPEC strain E2348/69, grown either in Ca2+-depleted medium or in Ca2+-containing medium, followed by immunoblot analysis, resulted in identification of these proteins as Tir, the translocated intimin receptor (Fig. 3A). In contrast to the 78-kDa form of Tir secreted by EPEC strain E2348/69, Tir of DA-EPEC strain 3431 has a molecular mass of approximately 90 kDa (Fig. 3A) (31). Again, Western blotting followed by immunostaining with specific EspB and EspD antibodies demonstrated that the secretion of EspB and EspD under Ca2+-depleted conditions was drastically reduced, as shown for DA-EPEC strain 3431 (Fig. 3A). Tir cannot be detected in the supernatant of the EPEC type III secretion mutant cfm 14-2-1 prepared under Ca2+-free conditions (data not shown). This clearly indicates that Tir secretion under calcium chelation conditions is a type III secretion-dependent process. Obviously, the secretion of Esp proteins and the secretion of Tir are affected in opposite ways by Ca2+, although these proteins are apparently secreted by the same TTSS. Depletion of Ca2+ in the culture media of EspB (UMD864) and EspD (UMD870) mutant strains of EPEC also increased the secretion of Tir, which indicates that under Ca2+-free conditions strong Tir secretion occurs independent of EspB or EspD (Fig. 3A).
FIG. 3.
Contrasting influence of Ca2+ on type III-secreted proteins. Precipitated supernatants of DA-EPEC strain 3431 and EPEC strains E2348/69, UMD864 (EPEC strain E2348/69 ΔespB), and UMD870 (EPEC strain E2348/69 ΔespD) grown with or without Ca2+ were separated by SDS-12.5% PAGE and subjected to Western blot analysis with anti-Tir antibody. For all strains, Ca2+ chelation resulted in increased Tir secretion. EspB and EspD were visualized in supernatants of DA-EPEC strain 3431 by probing the blot with specific polyclonal antisera. Compared to the EspB and EspD secretion of DA-EPEC grown with Ca2+, only residual secretion was detected under Ca2+-depleted conditions. The loaded supernatants from cultures of strains grown with and without Ca2+ were supernatants prepared from the same number of cells. (B) Whole-cell extracts of DA-EPEC strain 3431 and EPEC strain E2348/69 were analyzed by SDS-10% PAGE and Western blotting by using anti-Tir antibody. Tir is expressed by EPEC and DA-EPEC grown with or without Ca2+. The amount of intracellular Tir increased in bacteria grown with Ca2+. The loaded extracts were extracts prepared from 2 × 108 bacteria.
To compare Tir expression in strains propagated with and without Ca2+, we analyzed bacterial whole-cell extracts by SDS-PAGE and immunoblotting. Tir was expressed by EPEC and DA-EPEC under either type of culture conditions (Fig. 3B). However, the amount of Tir in whole-cell extracts derived from bacteria grown with Ca2+ was greater than the amount of Tir in cellular extracts of bacteria grown without Ca2+ (Fig. 3B). This indicates that in the presence of Ca2+ Tir accumulates in the bacteria; however, in the absence of Ca2+ the secretion of Tir increases.
Tir secreted in the presence of Ca2+ integrates into HeLa cell membranes.
EPEC strains translocate Tir into the host cell membrane, where it serves as the receptor for intimin (7, 27). Intimin binding is followed by induction of the characteristic actin-rich pedestals (45). As shown by immunofluorescence, the Tir protein derived from LEE-positive DA-EPEC strains (e.g., DA-EPEC strain 3431) colocalizes with accumulated actin (data not shown). Furthermore, cellular fractionation of DA-EPEC-infected HeLa cells confirmed the membrane localization of Tir (31). This indicates that Tir of DA-EPEC most likely has the same function as Tir of EPEC.
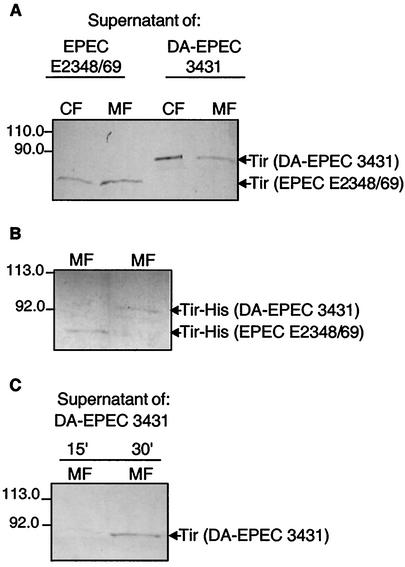
To determine whether Tir, which is secreted under Ca2+-free conditions, is also delivered into target cells, we incubated HeLa cells with bacterium-free, Tir-containing supernatants of EPEC strain E2348/69 or DA-EPEC strain 3431 which had been grown without Ca2+. Fractionation of cells was performed as described in Materials and Methods, and cytosolic fractions and Triton X-100-soluble membrane fractions were isolated and used for Western blot analysis. Potential cross-contamination of the membrane fractions with cytosolic proteins could be excluded by probing the blots with antibodies against the cytosolic protein kinase C (data not shown). As shown in Fig. 4A, Tir derived from EPEC strain E2348/69 and Tir derived from DA-EPEC strain 3431 were detected in the fractions containing Triton X-100-soluble membrane proteins of HeLa cells. Furthermore, following incubation with either DA-EPEC or EPEC supernatant we detected Tir in the cytosolic fractions of HeLa cells (Fig. 4A). In addition, we incubated HeLa cells with purified His-tagged Tir of EPEC strain E2348/69 and DA-EPEC strain 3431. Interestingly, Tir was also detected in membrane fractions of these cells (Fig. 4B). Furthermore, we determined the minimal incubation time needed to detect Tir of DA-EPEC strain 3431 in membrane fractions (Fig. 4C). As a control, HeLa cells were incubated for 2 h with glutathione S-transferase (GST). We could not detect GST in membrane fractions of these cells (data not shown), even though we used 10-fold more GST than Tir. This indicates that there is Tir-specific integration. Taken together, these results indicate that Tir can apparently be delivered by two different mechanisms to host cells. In an environment containing sufficient levels of Ca2+, Tir secretion and delivery into target cells are facilitated by EspA, EspB, and EspD (27). However, under Ca2+-free conditions, when no or only residual levels of EspA, EspB, and EspD are available, Tir is efficiently secreted and subsequently integrated directly into host cell membranes. Moreover, recombinant Tir is able to integrate into eukaryotic cell membranes. This clearly shows that this process occurs without the contribution of other factors that might be in the culture supernatant and, in contrast to Tir secretion, appears to be independent of the calcium level.
FIG. 4.
Tir delivery into HeLa cells under Ca2+-depleted conditions. HeLa cells were incubated for 15 or 30 min (C) or for 120 min (A and B) with bacterium-free supernatant of EPEC strain E2348/69 or DA-EPEC strain 3431 grown without Ca2+ (A and C) or recombinant His-tagged Tir (B). After repeated washing, the cells were fractionated as described in the text. Cytoplasmic (CF) and membrane (MF) fractions were separated by SDS-12.5% PAGE (A) or SDS-10% PAGE (B and C) and transferred to nitrocellulose membranes. The immunoblotting analysis was performed by using anti-Tir antibody.
DISCUSSION
The expression of the TTSS and the secretion of type III-secreted proteins of EPEC are influenced by various environmental conditions (3, 16, 26, 29, 46). Here, we investigated the influence of Ca2+ on the TTSS-mediated secretion of effector proteins derived from EPEC and DA-EPEC strains. Depending on the Ca2+ concentrations available in the growth medium, different effects on type III secretion of proteins were observed. Under Ca2+-depleted conditions the secretion of EspA, EspB, and EspD in DA-EPEC and EPEC strains was either completely eliminated (DA-EPEC strains B6 and 3431) or at least strongly reduced (EPEC strain E2348/69). Kenny et al. (26) reported a two- to fourfold reduction in Esp secretion under Ca2+-depleted conditions in EPEC and described the secretion of additional proteins. Here we identified one of these proteins as the translocated intimin receptor (Tir).
Recently, a type III secretion-dependent hemolytic activity based on the ability of the EspB and EspD proteins to form pores in target cell membranes has been described for EPEC and DA-EPEC (52; T. Ide, S. Laarmann, C. Wachter, C. Beinke, L. Greune, and M. A. Schmidt, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. B-327, 2000). As expected, the decrease in Esp secretion under Ca2+-depleted conditions also led to a decrease in hemolytic activity. The hemolytic activity of the supernatant of DA-EPEC grown under calcium-depleted conditions was also eliminated. In contrast, chelation of Ca2+ in the Esp-containing supernatant immediately before addition to the erythrocytes had only a marginal effect on the hemolytic activity. These results demonstrate that the influence of Ca2+ on the hemolytic activity is due to inhibition of Esp secretion and not to inhibition of Esp activity.
Transcription of the type III-secreted proteins in EPEC is under control of the integration host factor, which seems to function as a master switch for LEE genes, including tir, espA, espB, and espD (18, 35). The integration host factor activates expression of Ler, which positively regulates the expression of operons LEE2 to LEE5 of the LEE pathogenicity island, including the espA, espB, espD, and tir genes. Per, encoded on the EAF plasmid, which has been identified in EPEC strains but not in DA-EPEC strains, seems to function in fine-tuning the expression of ler (35). Thus, Esp expression and Tir expression are subject to the same transcriptional regulation. Our data suggest that EPEC and DA-EPEC strains are able to regulate the secretion of Esp independent of secretion of Tir.
In Yersinia spp. expression of type III-secreted proteins is coregulated with secretion of these proteins (21, 42). The control of Yop release is mediated by the secretion channels of the secretion machinery, which are opened under calcium-free conditions or by contact of the bacteria with a target cell (47). YopN is thought to function as a regulatory plug that blocks Yersinia type III secretion channels in the presence of calcium (14, 41, 47). However, such a regulatory protein has not been described for EPEC. Such a mechanism would also not explain the divergent secretion of Tir and Esp proteins in response to Ca2+. Moreover, in this study we show that in contrast to the calcium regulation of Yersinia, transcription of EPEC or DA-EPEC espA, espB, espD, or tir is apparently not influenced by calcium.
In Shigella, transcription and secretion of type III-secreted proteins are not coregulated (36). The Shigella type III secretion channels are kept shut by the IpaB/IpaD complex before cell contact (36, 38, 40). Ipa proteins accumulate in the cytoplasm and are protected from interaction and degradation by the chaperone IpgC (37). It is thought that after target cell contact the IpaB/IpaD block is released and Ipa secretion occurs.
The models describing the regulation of secretion in Yersinia and Shigella can obviously not explain the divergent secretion of Esp proteins and Tir. Nevertheless, it is possible that there are similar mechanisms that regulate secretion of Esp proteins and Tir in EPEC. The chaperones of Tir and of EspB and EspD might also be good candidates for molecules that are involved in regulation of a contrasting TTSS in EPEC. CesD is able to interact with EspD and is absolutely required for proper secretion of EspB and EspD. Its absence results in complete inhibition of EspD secretion and in a strong decrease in EspB secretion (51). Thus, EspB and EspD secretion could be regulated via CesD. Additionally, the Tir chaperone CesT might function as a regulator for Tir secretion under certain conditions (12). Studies are under way to analyze the role of these chaperones in the regulation of secretion.
Although the secretion pattern of TTSS-secreted proteins in EPEC and DA-EPEC bacteria is clearly influenced by Ca2+, this finding does not definitely prove that calcium is also the signal leading to the observed reduction in Esp secretion and induction of Tir secretion in vivo. Low calcium levels might mimic a signal generated by cell contact; this possibility has also been discussed for Yersinia type III secretion.
The mechanism by which Tir is integrated into host cell membranes has not been elucidated so far. Several studies have shown that at least EspB and EspA are involved in Tir delivery into host cell membranes after an EPEC infection (19, 27, 46) This led to the current model which suggests that Tir translocates in an EspA-, EspB-, and EspD-dependent manner into the host cytosol, where it is phosphorylated and subsequently integrated into the membrane (25). The presence of Tir in membrane fractions of HeLa cells after incubation with the supernatants of DA-EPEC and EPEC cultures grown under calcium-free conditions which contained Tir and no or only residual amounts of EspB or EspD proteins strongly suggests that there is an Esp- and contact-independent mechanism for Tir delivery into host cells. The possibility that there was bacterial contamination can be excluded as the supernatants were filter sterilized before use. In addition, we found that in contrast to Tir, no EspD and only very small amounts of EspB were detectable in Western blotting experiments with supernatants of DA-EPEC grown under calcium-free conditions. The amounts of EspB and EspD in supernatants of EPEC strain E2348/69 grown in medium without calcium were also reduced. It is possible that small amounts of Esp proteins are sufficient for Tir delivery to host cells, as reported by Kenny et al. (27). However, as we demonstrated that type III-secreted Tir is able to integrate into host membranes in the absence of bacteria, type III translocation system-dependent Tir insertion into host membranes under Ca2+-free conditions is rather unlikely. In accordance with these results, we detected Tir in membrane fractions of HeLa cells which were incubated with purified His-tagged Tir from EPEC strain E2348/69 and DA-EPEC strain 3431. Based on the evidence obtained in this study, we propose that under certain environmental conditions, which can be mimicked by calcium chelation, Tir is able to integrate directly into host cell membranes by a different mechanism. This proposal is also supported by data obtained by Rabinowitz et al. (44), who identified an EspB secretion mutant which was still able to induce Tir-dependent A/E activity. DeVinney et al. subsequently reported that only EspB secretion is reduced in this mutant strain (8). However, the mutant strain is able to deliver Tir to the host cell in the absence of detectable EspA, EspB, and EspD secretion, and it was suggested by the investigators that Tir translocation and A/E lesion formation can be uncoupled from Esp secretion. Furthermore, it was demonstrated by DeVinney et al. (9) that EHEC which expressed EPEC Tir Y474F (a nonphosphorylated EPEC Tir mutant form) induce pedestals in the host cell. However, as there are high levels of sequence divergence between the EspB proteins and EspD proteins of EHEC and EPEC (13), passage through the EHEC TTSS translocon in the cytoplasmic membrane seems to be not very likely.
Interestingly, only the unmodified Tir with a molecular mass of 72 to 78 kDa and not the modified 90-kDa form of EPEC Tir was detectable in the membrane fractions. In recent studies, other workers also identified unmodified Tir in membrane fractions of eukaryotic cells, which may indicate that this protein is translocated into the membrane without a cytosolic intermediate (25). It has been shown by Gauthier et al. (19) that phosphorylation of tyrosine 474 of Tir is essential for actin rearrangement but not for membrane integration. Furthermore, Tir was detected in the membrane fraction as early as 1.5 h after EPEC infection of HeLa cells but appeared in the cytoplasmic fraction only at very late time points. This also suggests that Tir may be translocated directly into the membrane (19). Our data favor a mechanism for direct Tir insertion into the host membrane without a need for a prior cytosolic intermediate. However, we also detected Tir in cytosolic fractions of HeLa cells. Because of the absence of direct bacterial contact and the weak Esp secretion under calcium-free conditions, the possibility of TTSS-dependent injection of Tir can be excluded. Therefore, the findings might be attributed to possible contamination of the cytosolic fraction with membrane-bound Tir during the isolation procedure.
Esp secretion by EPEC is induced not only by calcium but also by contact with host cells (53). The translocation of, for example, EspB is strongly promoted by the attachment factors of EPEC, intimin and bundle-forming pili. Both factors are necessary for efficient translocation of effector proteins. Thus, taking this into account together with the findings presented here, we propose the following model for alternative Tir delivery. (i) Tir, once secreted by the type III secretion machinery, is able to integrate independent of Esp proteins and thus independent of the type III translocon into host cell membranes. (ii) Subsequent binding of Tir to intimin mediates the intimate attachment between eukaryotic cell and bacterium and (iii) thereby induces a signal which leads to Esp secretion and formation of the type III translocon.
Alteration of the intracellular calcium content in host cells which could be associated with an EPEC infection has been discussed in a very controversial manner. Studies on host signal transduction events induced by EPEC showed that there was an increase in the intracellular Ca2+ level in infected cells, which was dependent on functional type III secretion and intimate attachment (2, 11). The binding of intimin and Tir induces host signaling events, such as the tyrosine phosphorylation of phospholipase Cγ-1, which leads to inositol triphosphate and Ca2+ fluxes (28). In contrast, Bain et al. were not able to detect an increase in the intracellular Ca2+ level in infected cells during an EPEC infection (1). However, our results support the idea that calcium may play a role in EPEC pathogenesis.
In conclusion, our findings strongly support the hypothesis that there is a mechanism for direct Tir delivery into host membranes which is independent of type III translocation but not type III secretion. Additionally, the existence of conditions which increase Tir secretion but result in reduced or even no Esp secretion raises new questions concerning the mechanism of such divergent regulation of type III secretion in EPEC and DA-EPEC.
Acknowledgments
We are indebted to J. B. Kaper (Baltimore, Md.) for providing EPEC strain E2348/69, to M. Donnenberg (Baltimore, Md.) for providing strains UMD864 and UMD870 and EPEC strain E2348/69 cfm 14-2-1, and to F. Ebel (Munich, Germany) for providing the anti-Tir antibody. Furthermore, we thank D. Tapadar (ZMBE, Münster, Germany) for critical reading of the manuscript.
This work was supported in part by grants GRK 234 and SFB293 B5 from the Deutsche Forschungsgemeinschaft.
Editor: V. J. DiRita
REFERENCES
- 1.Bain, C., R. Keller, G. K. Collington, L. R. Trabulsi, and S. Knutton. 1998. Increased levels of intracellular calcium are not required for the formation of attaching and effacing lesions by enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 66:3900-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, T. J., W. Ward, A. Aitken, S. Knutton, and P. H. Williams. 1991. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect. Immun. 59:1599-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beinke, C., S. Laarmann, C. Wachter, H. Karch, L. Greune, and M. A. Schmidt. 1998. Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins. Infect. Immun. 66:528-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secretion of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burland, V., Y. Shao, N. T. Perna, G. Plunkett, H. J. Sofia, and F. R. Blattner. 1998. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 26:4196-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniell, S. J., N. Takahashi, R. Wilson, D. Friedberg, I. Rosenshine, F. P. Booy, R. K. Shaw, S. Knutton, G. Frankel, and S. I. Aizawa. 2001. The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell Microbiol. 3:865-871. [DOI] [PubMed] [Google Scholar]
- 7.Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol. Microbiol. 28:463-474. [DOI] [PubMed] [Google Scholar]
- 8.Devinney, R., I. Nisan, S. Ruschkowski, I. Rosenshine, and B. B. Finlay. 2001. Tir tyrosine phosphorylation and pedestal formation are delayed in enteropathogenic Escherichia coli sepZ::TnphoA mutant 30-5-1(3). Infect. Immun. 69:559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVinney, R., J. L. Puente, A. Gauthier, D. Goosney, and B. B. Finlay. 2001. Enterohaemorrhagic and enteropathogenic Escherichia coli use a different Tir-based mechanism for pedestal formation. Mol. Microbiol. 41:1445-1458. [DOI] [PubMed] [Google Scholar]
- 10.Donnenberg, M. S. 1995. Enteropathogenic Escherichia coli, p. 709-726. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, Ltd., New York, N.Y.
- 11.Dytoc, M., L. Fedorko, and P. M. Sherman. 1994. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology 106:1150-1161. [DOI] [PubMed] [Google Scholar]
- 12.Elliott, S. J., S. W. Hutcheson, M. S. Dubois, J. L. Mellies, L. A. Wainwright, M. Batchelor, G. Frankel, S. Knutton, and J. B. Kaper. 1999. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol. Microbiol. 33:1176-1189. [DOI] [PubMed] [Google Scholar]
- 13.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 14.Forsberg, A., A. M. Viitanen, M. Skurnik, and H. Wolf-Watz. 1991. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5:977-986. [DOI] [PubMed] [Google Scholar]
- 15.Foubister, V., I. Rosenshine, M. S. Donnenberg, and B. B. Finlay. 1994. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect. Immun. 62:3038-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis, M. S., H. Wolf-Watz, and A. Forsberg. 2002. Regulation of type III secretion systems. Curr. Opin. Microbiol. 5:166-172. [DOI] [PubMed] [Google Scholar]
- 17.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 18.Friedberg, D., T. Umanski, Y. Fang, and I. Rosenshine. 1999. Hierarchy in the expression of the locus of enterocyte effacement genes of enteropathogenic Escherichia coli. Mol. Microbiol. 34:941-952. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier, A., M. de Grado, and B. B. Finlay. 2000. Mechanical fractionation reveals structural requirements for enteropathogenic Escherichia coli Tir insertion into host membranes. Infect. Immun. 68:4344-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15:5812-5823. [PMC free article] [PubMed] [Google Scholar]
- 21.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ide, T., S. Laarmann, L. Greune, H. Schillers, H. Oberleithner, and M. A. Schmidt. 2001. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaper, J. B. 1998. EPEC delivers the goods. Trends Microbiol 6:169-172. (Discussion, 6:172-173.) [DOI] [PubMed]
- 25.Kenny, B. 1999. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol. 31:1229-1241. [DOI] [PubMed] [Google Scholar]
- 26.Kenny, B., A. Abe, M. Stein, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 65:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 28.Kenny, B., and B. B. Finlay. 1997. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-gamma1. Infect. Immun 65:2528-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenny, B., and B. B. Finlay. 1995. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc. Natl. Acad. Sci. USA 92:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenny, B., L. C. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20:313-323. [DOI] [PubMed] [Google Scholar]
- 31.Laarmann, S. 1999. Ph.D. thesis. Westfälische Wilhelms-Universität, Münster, Germany.
- 32.Levine, M. M., and R. Edelmann. 1984. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol. Rev. 6:31-51. [DOI] [PubMed] [Google Scholar]
- 33.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 34.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellies, J. L., S. J. Elliott, V. Sperandio, M. S. Donnenberg, and J. B. Kaper. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 36.Menard, R., P. Sansonetti, and C. Parsot. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13:5293-5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menard, R., P. Sansonetti, C. Parsot, and T. Vasselon. 1994. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79:515-525. [DOI] [PubMed] [Google Scholar]
- 38.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neyt, C., and G. R. Cornelis. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33:971-981. [DOI] [PubMed] [Google Scholar]
- 40.Parsot, C., R. Menard, P. Gounon, and P. J. Sansonetti. 1995. Enhanced secretion through the Shigella flexneri Mxi-Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol. Microbiol. 16:291-300. [DOI] [PubMed] [Google Scholar]
- 41.Persson, C., R. Nordfelth, A. Holmstrom, S. Hakansson, R. Rosqvist, and H. Wolf-Watz. 1995. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18:135-150. [DOI] [PubMed] [Google Scholar]
- 42.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 43.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 44.Rabinowitz, R. P., L. C. Lai, K. Jarvis, T. K. McDaniel, J. B. Kaper, K. D. Stone, and M. S. Donnenberg. 1996. Attaching and effacing of host cells by enteropathogenic Escherichia coli in the absence of detectable tyrosine kinase mediated signal transduction. Microb. Pathog. 21:157-171. [DOI] [PubMed] [Google Scholar]
- 45.Rosenshine, I., M. S. Donnenberg, J. B. Kaper, and B. B. Finlay. 1992. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 11:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenshine, I., S. Ruschkowski, and B. B. Finlay. 1996. Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature, and protein synthesis upon contact with epithelial cells. Infect. Immun. 64:966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Straley, S. C., E. Skrzypek, G. V. Plano, and J. B. Bliska. 1993. Yops of Yersinia spp. pathogenic for humans. Infect. Immun. 61:3105-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wachter, C., C. Beinke, M. Mattes, and M. A. Schmidt. 1999. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 31:1695-1707. [DOI] [PubMed] [Google Scholar]
- 51.Wainwright, L. A., and J. B. Kaper. 1998. EspB and EspD require a specific chaperone for proper secretion from enteropathogenic Escherichia coli. Mol. Microbiol. 27:1247-1260. [DOI] [PubMed] [Google Scholar]
- 52.Warawa, J., B. B. Finlay, and B. Kenny. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect. Immun. 67:5538-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolff, C., I. Nisan, E. Hanski, G. Frankel, and I. Rosenshine. 1998. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 28:143-155. [DOI] [PubMed] [Google Scholar]