Abstract
Burkholderia pseudomallei, a facultatively intracellular pathogen, is a flagellated and motile gram-negative bacterium and is the causative agent of melioidosis in humans. Flagella are commonly recognized as important virulence determinants expressed by bacterial pathogens since the motility phenotype imparted by these organelles often correlates with the ability of an organism to cause disease. We used a virulent isolate of B. pseudomallei, KHW, to construct an isogenic deletion mutant with a mutation in the flagellin gene (fliC) by gene replacement transposon mutagenesis. The KHWΔfliCKm mutant was aflagellate and nonmotile in semisolid agar. The isogenic KHWΔfliCKm mutant was not impaired in terms of the ability to invade and replicate in cultured human lung cells compared with the wild type. It was also equally virulent in slow-killing assays involving Caenorhabditis elegans, but it was avirulent during intranasal infection of BALB/c mice. Very few bacteria, if any, were isolated from the lungs and spleens of KHWΔfliCKm-infected mice. In contrast, the bacterial loads in the lungs and spleens were similar in mice infected with KHW and in mice infected with the complemented mutant, KHWΔfliCKm/pUCP28TfliC. Unlike the Syrian hamster or diabetic rat models of infection, the B. pseudomallei flagellin was also a virulence factor during intraperitoneal infection of BALB/c mice. In this study, all animals infected with KHWΔfliCKm remained healthy and did not succumb to disease regardless of the route of infection. The flagellum is therefore an important and necessary virulence determinant of B. pseudomallei during intranasal and intraperitoneal infection of mice.
Infection by Burkholderia pseudomallei occurs via inhalation of contaminated dust or when contaminated soil comes in contact with an abraded area of the skin (2). The infection commonly begins in the lungs, where a pus abscess may form, or it may spread from the skin through the blood to affect the heart, brain, liver, kidneys, joints, and eyes. The pathogenesis of melioidosis is poorly defined. B. pseudomallei can invade both cultured phagocytic and nonphagocytic cells. In these cells, bacterial invasion is followed by intracellular multiplication and induction of cell fusion and multinucleate giant cell formation. Photomicrographs have demonstrated that B. pseudomallei can induce the formation of peripheral membrane protrusions in both phagocytic and nonphagocytic cells, which are similar to the protrusions found in cells infected by Listeria monocytogenes and Shigella flexneri (13). B. pseudomallei-infected cells also show actin rearrangement that occurs at only one polar end of the bacillus. The ability of B. pseudomallei to adopt a facultatively intracellular existence may be an important property in the pathogenesis of both acute and chronic infections (13).
B. pseudomallei is also capable of producing secreted and cell-associated antigens, but the roles of these products in the pathogenesis of disease remain unclear (2). The flagella and motility, as well as the resistance of the organism to the bactericidal action of normal human serum, are believed to play roles in the ability of the bacterium to disseminate from sites of localized infection, such as the lungs or skin, to virtually any other organ of the body via the blood circulatory system (5, 11).
Several genes involved in motility have been identified by transposon mutagenesis of B. pseudomallei. Among these is fliC, which encodes flagellin (5). In B. pseudomallei, the fliC gene and the flagellin protein were detected in 108 B. pseudomallei isolates obtained from human patients, animals, and soil (Chua, unpublished data). Antibodies raised against the B. pseudomallei flagellin markedly reduced the motility of the bacterium and provided passive protection against B. pseudomallei infection in animal models (1). However, no attenuation of virulence was observed between the fliC mutant and the wild-type parental strain when diabetic infant rats or Syrian hamsters were infected intraperitoneally, suggesting that flagella and/or motility are probably not virulence determinants in the pathogenesis of the bacteria in these models (5).
In several other bacterial pathogens, such as Salmonella enterica serovar Typhimurium and Vibrio cholerae, virulence has been correlated with flagella and bacterial motility, but it is not clear how flagella function as virulence factors (3, 10). Flagellar motility has been reported to enhance the invasion of host cells by Campylobacter jejuni, Proteus mirabilis, Vibrio anguillarum, and other pathogenic species (16, 18, 28). For example, a nonmotile mutant of Burkholderia cepacia with a defective component of the motor-switch complex of the flagellar basal body was found to be less invasive in lung epithelial cells (23). Flagellum-mediated motility has been implicated in the pathogenesis of B. cepacia because it facilitates penetration of the host epithelial cell barriers and contributes to the onset of systemic spread of the organism (23).
In this study, we constructed an isogenic fliC deletion mutant of B. pseudomallei by targeted gene replacement via homologous recombination. We compared the cell invasion properties and virulence of this mutant with the cell invasion properties and virulence of an isogenic wild-type B. pseudomallei strain. In order to ascertain the role of flagella as virulence determinants in B. pseudomallei, we used intranasally infected BALB/c mice as an animal model of infection (15). We also included virulence testing with Caenorhabditis elegans as an alternative model system to evaluate its applicability for investigating the role of flagella in B. pseudomallei pathogenesis (9).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains and plasmids are listed in Table 1. B. pseudomallei isolate KHW was obtained from the collection of E. H. Yap at the Department of Microbiology, National University of Singapore. Escherichia coli strains were cultured in Luria-Bertani broth or on Luria-Bertani agar at 37°C, while bacteria carrying pUCP28T constructs were cultured in the presence of 25 μg of trimethoprim per ml. B. pseudomallei was cultured on tryptic soy agar (TSA) or in tryptic soy broth (TSB) (Oxoid, Basingstoke, England). KHWΔfliCKm was cultured in TSB containing 200 μg of kanamycin per ml, while KHWΔfliCKm/pUCP28TfliC was cultured in TSB containing 100 μg of trimethoprim per ml.
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Genotype and/or phenotype | Source or reference |
|---|---|---|
| Escherichia coli strains | ||
| KL98 | Hfr strain, thi Sms | P. Oliver |
| DH5αλpir | λpir lysogen of DH5α for replication of oriR6K, oriT, and mob region of RP4, Kms | N. Judson, GIBCO-BRL |
| Sm10 | Mobilizing strain, transfer genes of RP4 integrated in chromosome | 21 |
| Burkholderia pseudomallei strains | ||
| KHW | Clinical strain | E. H. Yap |
| KHWΔfliCKm | Derivative of KHW, with central fragment of the fliC gene replaced by a 2.3-kb kanamycin cassette from pUTKm | This study |
| Plasmids | ||
| pJQ200mp18 | Suicide vector, oriT and mob region from RP4 and sacB from Bacillus subtilis, Gmr | 19 |
| pJQ200mp18fliCKm | pJQ200mp18 with 3.4-kb kanamycin cassette-disrupted B. pseudomallei fliC sequence inserted at the SmaI site | This study |
| pUTKm | Source of 2.3-kb Tn903-derived Kmr cassette | 4 |
| pUCP28T | Broad-host-range vector; oriT and mob region from RP4, Tpr | H. Schweizera |
| pUCP28TfliC | Contains a 1.5-kb full-length B. pseudomallei fliC gene and promoter inserted into the SmaI site by poly(I) tailing | This study |
See reference 27.
Cell lines.
Human lung carcinoma epithelial cell line A549 was a kind gift from Vincent Chow at the National University of Singapore and was maintained at 37°C in the presence of 5% CO2 in Dulbecco's modified Eagle's medium (Sigma Chemical Co., St. Louis, Mo.), supplemented with 10% fetal bovine serum (Gibco, Grand Island, N.Y.) and a mixture containing 100 U of penicillin per ml and 0.1 mg of streptomycin per ml (Sigma).
Construction of the KHWΔflicKm mutant and the fliC complement plasmid pUCP28TfliC.
KHW, a virulent strain of B. pseudomallei isolated from a human patient, was selected as the parent strain for the construction of fliC deletion mutants. Briefly, the KHWΔfliCKm mutant was constructed by cloning the full-length B. pseudomallei fliC gene into the suicide vector pJQ200mp18 (19) and replacing the central 0.7-kb PstI fragment of the fliC gene (nucleotide positions 294 to 979 of the fliC gene sequence [GenBank accession no. U82287]) with a 2.3-kb kanamycin cassette from plasmid pUTKm (GenBank accession no. AF102233) (4). The pJQ200mp18ΔfliCKm construct, transformed into E. coli DH5αλpir, was confirmed by DNA sequencing with primers KmF (5′TCAAGGATCTGGATTTC) and KmR (5′TTGTTATCGCAATAGTTG). The construct was then introduced into B. pseudomallei KHW by conjugation, and reciprocal recombinants were selected by plating the conjugation mixture on TSB supplemented with 200 μg of kanamycin per ml and 5% sucrose. Four fliC gene knockout mutants, mutants 5, 6, 19, and 20, were obtained. These KHWΔfliCKm mutants were characterized further with respect to motility, flagellum synthesis, and PCR, and Western blotting was used for immunodetection of flagellin protein.
The full-length B. pseudomallei fliC gene and its promoter were amplified from genomic DNA of KHW by using PCR primers Promoter1F (5′ AATTCAGCCCGCCATCATAA) and FL8 (5′CGTTCGCTACACGTTATTGC). The 1.5-kb PCR product was ligated into the poly(T)-tailed, broad-host-range vector pUCP28T and was transformed into E. coli Sm10. Transformants were selected on Luria agar plates containing 25 μg of trimethoprim per ml, and the presence of the fliC insert was confirmed by PCR by using fliC-specific primers Promoter1F and FL8, as well as by DNA sequencing (data not shown). The pUCP28TfliC construct was introduced into KHWΔfliCKm mutant 6 via conjugation. Exconjugants were selected on TSA plates containing 100 μg of trimethoprim per ml and 10 μg of streptomycin per ml. Three positive clones were obtained.
Northern blotting.
A 1,521-bp open reading frame encoding a putative protein with homology to the flagellar capping protein, FliD, of Pseudomonas aeruginosa was identified 150 bp downstream of the B. pseudomallei fliC gene. The fliD probe for Northern blotting was generated by PCR by using primers FliDF (5′GGAGTCGCCGAGGAGCGGCCC) and FliDR (5′CGGCACGCTGAAGGCCGCG). The 826-bp product was [α-32P]dCTP labeled by using Ready-to-Go DNA labeling beads (Pharmacia Biotech Inc., Uppsala, Sweden). Northern blotting was performed by using 5-μg portions of total RNA isolated from KHW, KHWfliCKm mutants, and DH5αλpir according to a procedure described by Sambrook et al. (20).
PCR.
PCR were performed by using Tth DNA polymerase (Biotools, Madrid, Spain) in 1.5 mM MgCl2 with a PTC-100 thermocycler (MJ Research Inc., Boston, Mass.). The cycling parameters for amplification with the fliC-specific primers FlaHisF (5′TCAGGATCCCTCGGAATCAACAGCAACATT) and FlaHisR (5′CAGAAGCTTTTATTGCAGGAGCTTCAGCAC) were as follows: 1 cycle of denaturation at 94°C for 3 min and 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 3 min, followed by 1 cycle of 72°C for 10 min. The parameters used for amplification with the FliDF and FliDR primers were similar except that they included 30 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min.
DNA sequencing.
DNA sequencing was performed with the ABI BigDye dye terminator cycle sequencing reagents (Perkin-Elmer Corp., Foster City, Calif.), and sequences were analyzed with an Applied Biosystems ABI 377 automated DNA sequencer.
Immunodetection of flagellin protein by Western blotting.
Five-microgram portions of total bacterial proteins prepared from overnight broth cultures of KHW, KHWΔfliCKm, KHWΔfliC complemented with wild-type fliC, and E. coli DH5α and 0.1 μg of recombinant B. pseudomallei FliC protein were electrophoresed by performing sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 4% polyacrylamide stacking and 10% polyacrylamide resolving gels and were electroblotted onto a nitrocellulose membrane. Western blot hybridization was performed as described by Towbin et al. (24) by using 20 mM Tris-HCl (pH 7.5)-500 mM NaCl-0.05% Tween 20 (TBST) containing 10% skim milk as the blocking reagent and a 1:5,000 dilution of mouse polyclonal antiflagellin antibodies as the primary antibody. The antiflagellin antibodies were pooled from five mice, each of which had been intranasally immunized with 20 μl (1 μg/μl) of His-tagged recombinant B. pseudomallei FliC per dose. Booster doses were given 14 and 28 days after the initial dose, and the serum was collected 1 week after the final booster injection. After 1 h of incubation with the primary antibody, the membrane was washed three times with TBST, a 1:2,000 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Amersham Pharmacia Biotech, Buckinghamshire, England) was added, and the preparation was incubated for 1 h. The membrane was washed again and immersed for 1 min in ECL Western blotting detection reagent (Amersham) for the detection of bound horseradish peroxidase by autoradiography.
Motility assay.
KHWΔfliCKm mutants were screened to determine their motility phenotypes by culturing 2-μl overnight broth cultures of individual clones in TSB with 0.3% agar containing 5% sucrose and 200 μg of kanamycin per ml. The plates were incubated for 20 h at 37°C, and motility was assessed qualitatively by examining the circular swarm formed by the growing motile bacterial cells.
Cell invasion assay.
Bacterial invasion of A549 cells was investigated by using the method described by Elsinghorst (7), except for the following modifications. Extracellular bacteria were killed by adding 200 μg of kanamycin per ml or 50 μg of tetracycline per ml instead of gentamicin, as KHW is resistant to gentamicin. Bacterial cells were cultured in TSB to the mid-log phase (optical density at 600 nm, 0.6), washed, and resuspended in an equal volume of 0.85% (wt/vol) NaCl. Twenty-five-microliter portions were added to confluent monolayers of eukaryotic cells (5 × 105 cells per well) in 24-well tissue culture plates. After centrifugation at 165 × g for 5 min and incubation at 37°C in the presence of 5% CO2 for 2 h, each monolayer was washed three times with phosphate-buffered saline (PBS), 1.5 ml of fresh culture medium containing 200 μg of kanamycin per ml or 50 μg of tetracycline per ml was added, and the preparation was incubated for an additional 2 or 24 h to kill the extracellular bacteria. The monolayer was then washed three times with PBS and lysed with 1 ml of 0.1% Triton X-100 (Sigma) before serial dilutions of the cell lysate were plated on TSA plates. The numbers of internalized bacteria obtained 2 and 24 h after infection represented the initial entry of bacteria and intracellular bacterial replication, respectively. All quantitative invasion assays were performed in triplicate wells. A noninvasive strain of E. coli K-12, KL98, was used as a negative control.
Virulence testing with C. elegans.
Bacterial virulence was tested by quantifying slow killing of C. elegans strain N2 (Bristol) by the method described by Gan et al. (9). Slow killing was defined as the contact-dependent killing of C. elegans on NG agar. Briefly, adult worms at mixed stages were floated from an agar plate by using 2 ml of M9 buffer, and 60 to 80 worms were seeded onto each NG agar plate inoculated with 20 μl of an overnight culture of B. pseudomallei. The droplet of bacteria in each plate was allowed to dry by leaving the plates at room temperature for 2 h before addition of the worms. The plates were incubated at room temperature and examined for live worms after every 24 h for 72 h. Duplicate experiments were performed for each trial, and a total of three trials were carried out for each test bacterium. A worm was considered dead when it no longer responded to touch.
Virulence testing with BALB/c mice.
Mice were intranasally infected with KHW, KHWΔfliCKm mutant 6, or the mutant complemented with wild-type fliC (KHWΔfliCKm/pUCP28TfliC). After the mice were anesthetized with a combination of Hypnorm and Dormicum, 20 μl of bacteria in PBS was intranasally inoculated through one nostril of each mouse. In the experiments used to determine the 50% lethal dose (LD50) for intranasal infection by the KHWΔfliCKm mutant, five animals per group were infected with doses ranging from 8,505 to 35 CFU with serial threefold dilutions. Mice were monitored for 20 days. For intraperitoneal infection with the KHWΔfliCKm mutant, six animals per group were used, and the doses ranged from 13,500 to 500 CFU with serial threefold dilutions. The mice were monitored for 30 days.
Electron microscopy of flagella.
One loopful of bacteria cultured on motility agar was scraped off and fixed for 2 h in PBS containing 2.5% glutaraldehyde (Agar Scientific, Stansted, United Kingdom). After a brief centrifugation and resuspension of the bacterial cells in PBS, a copper grid (Agar Scientific, Ltd., Essex, United Kingdom) was placed on a drop of bacterial suspension for 1 min. The grid was then dried and placed on a drop of a bacitracin solution (30 mg/ml; Sigma) for another 1 min and dried. The bacteria were negatively stained for 1 min by adding a drop of 1% phosphotungstate (pH 6.0; BDH, Poole, United Kingdom). The dried samples were examined with an EM208 S scanning electron microscope (Philips, Eindhoven, The Netherlands) at an acceleration voltage of 80 kV with calibrated magnification.
Statistical analysis.
Student's t test was used to determine if there was a statistically significant difference (P < 0.05) between the mean invasion frequencies for the wild type and the KHWΔfliCKm mutant, and a paired Student's t test was performed to check for any statistically significant differences in intracellular replication between the wild-type strain KHW and the KHWΔfliCKm mutant.
RESULTS
Construction and characterization of the KHWΔfliCKm mutant.
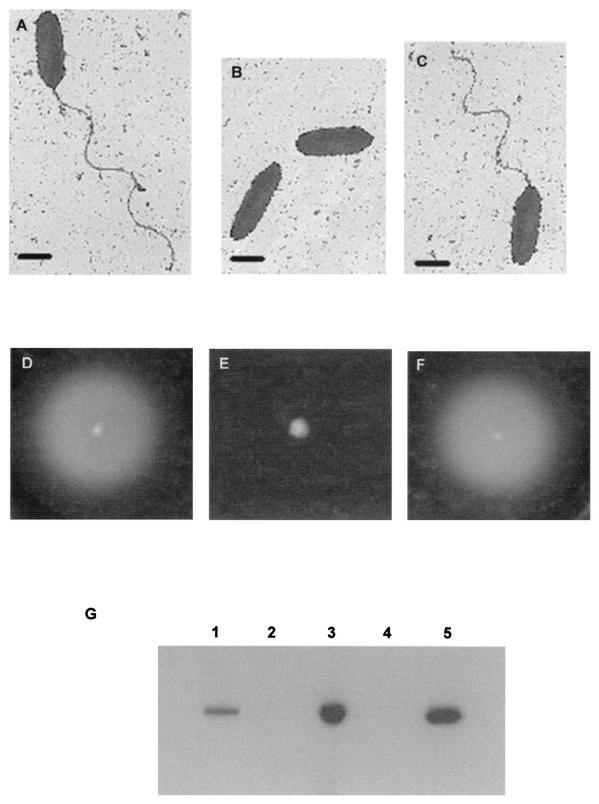
B. pseudomallei isolate KHW was chosen for construction of an isogenic strain that differed only by deletion of fliC because it was previously shown to be highly invasive in cell invasion assays and to be virulent in killing assays involving C. elegans and BALB/c mice (9, 15). Isolate KHW was identified as B. pseudomallei by using the API 20NE biochemical test, as well as 16S rRNA sequence analysis (data not shown). A 2.3-kb kanamycin resistance cassette was used to replace the 0.7-kb central portion of the B. pseudomallei fliC gene and to provide a selectable marker for the fliC gene disruption cassette on the suicide vector pJQ200mp18. This was necessary because B. pseudomallei KHW is inherently gentamicin resistant. Insertion of the kanamycin resistance cassette was confirmed by sequencing with primers KmF and KmR from the ends of the kanamycin cassette into the flanking fliC sequences. Four fliC gene knockout mutants, KHWΔfliCKm mutants 5, 6, 19, and 20, were obtained after DH5α:λpir/pJQ200mp18ΔfliCKm was conjugated with KHW and the recombinants were selected on TSA containing 200 μg of kanamycin per ml and 5% sucrose. We chose KHWΔfliCKm mutant 6 for further study. Unlike KHW, the KHWΔfliCKm mutant did not produce flagella (Fig. 1A and B). It also was nonmotile on 0.3% semisolid agar, in contrast to wild-type strain KHW (Fig. 1D and E). Unlike wild-type strain KHW, the 39-kDa flagellin protein was absent in Western blots of total cell lysates prepared from the KHWΔfliCKm mutant (Fig. 1G). The phenotypes of the mutant were consistent with a fliC gene knockout mutation.
FIG. 1.
Phenotypic characterization of wild-type strain KHW, the KHWΔfliCKm mutant, and the KHWΔfliCKm/pUCP28TfliC complemented mutant with respect to the presence of flagella, motility, and flagellin protein. (A to C) Electron micrographs showing flagella of KHW (A), KHWΔfliCKm mutant 6 (B), and the KHWΔfliCKm/pUCP28TfliC complemented mutant (C). Bars = 1 μm. (D to F) Motility assays for KHW (D), KHWΔfliCKm mutant 6 (E), and the KHWΔfliCKm/pUCP28TfliC complemented mutant (F) on semisolid agar, as described in Materials and Methods. (G) Western blot analysis of flagellin protein in total cell lysates prepared from E. coli DH5α:λpir (lane 2), KHW (lane 3), KHWΔfliCKm mutant 6 (lane 4), and the KHWΔfliCKm/pUCP28TfliC complemented mutant (lane 5). Lane 1 contained 0.1 μg of recombinant B. pseudomallei FliC protein. Five-microgram portions of total bacterial proteins prepared from overnight broth cultures of KHW, KHWΔfliCKm, the KHWΔfliCKm/pUCP28TfliC complemented mutant, and E. coli DH5α were separated on a sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis gel and immunoblotted with polyclonal mouse antiflagellin antibodies as described in Materials and Methods. The bands correspond to the 39-kDa flagellin protein.
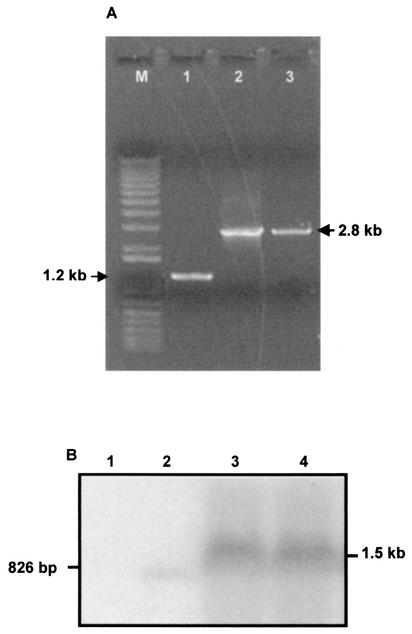
The presence of the gene replacement mutation in KHWΔfliCKm mutant 6 was verified by PCR by using fliC-specific primers flanking the replacement sequence. A 2.8-kb PCR product was obtained from the KHWΔfliCKm mutant, compared with the 1.2-kb PCR product obtained from KHW (Fig. 2A). This was consistent with reciprocal replacement of a central 0.7-kb fragment of fliC with a 2.3-kb kanamycin resistance cassette. In addition, we ascertained that the gene replacement with the kanamycin resistance cassette did not result in a polar mutation by comparing the expression of an adjacent downstream gene, which showed sequence homology to fliD of P. aeruginosa, in KHW and the mutant. In a Northern blot analysis of total RNA isolated from the KHWΔfliCKm mutant and KHW we detected fliD mRNA in both strains (Fig. 2B). Thus, the phenotypes described for the KHWΔfliCKm mutant were not due to altered expression of downstream genes.
FIG. 2.
(A) Verification of the construction of the B. pseudomallei KHWΔfliCKm mutant by PCR performed with fliC-specific primers FlaHisF and FlaHisR, as described in Materials and Methods. PCR products that were 2.8 kb long, corresponding to the disrupted fliC gene, were obtained for E. coli DH5α:λpir/pJQ200mp18fliCKm (lane 2) and KHWΔfliCKm mutant 6 (lane 3). A 1.2-kb PCR product corresponding to the fliC gene was obtained for wild-type strain KHW (lane 1). A 1-kb Plus DNA ladder (Gibco-BRL, Rockville, Md.) was used as the molecular size markers (lane M). (B) Northern blot analysis of fliD mRNA in wild-type strain KHW and the KHWΔfliCKm mutant. Five-microgram portions of total RNA were isolated from DH5α:λpir (lane 1), the 826-bp fliD PCR product (lane 2), wild-type KHW (lane 3), and KHWΔfliCKm mutant 6 (lane 4) and separated by formaldehyde RNA gel electrophoresis (Stratagene, La Jolla, Calif.). The probe used was a 826-bp [α-32P]dCTP-labeled B. pseudomallei fliD PCR product.
Furthermore, by complementation with a plasmid expressing wild-type fliC (pUCP28TfliC), we also ascertained that the phenotype observed for the KHWΔfliCKm mutant was not due to a second-site mutation. The complemented mutant, KHWΔfliCKm/pUCP28TfliC, had flagella (Fig. 1C), was motile (Fig. 1F), and synthesized wild-type flagellin (Fig. 1G).
Bacterial internalization and intracellular multiplication in cultured mammalian cells.
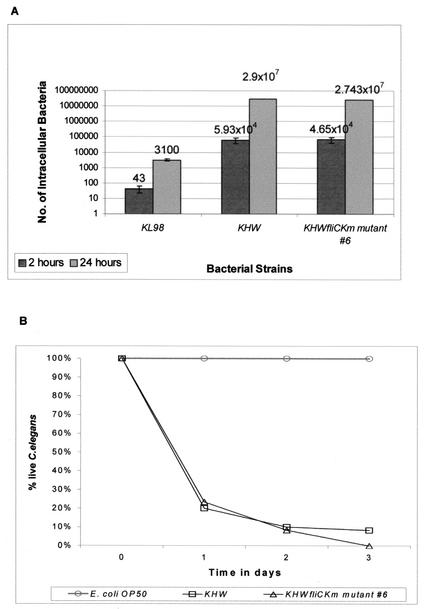
KHW and KHWΔfliCKm mutant 6 were equally invasive for A549 human lung carcinoma epithelial cells (Fig. 3A). There was also no difference between the abilities of the wild-type strain B. pseudomallei KHW and the KHWΔfliCKm mutants to replicate in A549 (Fig. 3A). The noninvasive E. coli K-12 strain KL98 was included as a negative control in these studies. Both the Student t test and paired Student's t tests showed that there was a significant difference (P < 0.05).
FIG. 3.
(A) Invasion of A549 cell monolayers and intracellular replication of B. pseudomallei KHW and KHWΔfliCKm mutant 6. Internalization was determined 2 h after exposure of the cell monolayers to the bacteria (dark gray bars). There was no significant difference between the invasiveness and intracellular replication of wild-type strain KHW and the invasiveness and intracellular replication of the mutant. Intracellular replication was determined by counting the number of intracellular bacteria 24 h after the initial exposure of the cell monolayers to the bacteria (light gray bars). The noninvasive E. coli strain KL98 was used as a negative control in these assays. (B) Role of fliC in C. elegans pathogenesis. B. pseudomallei KHW and KHWΔfliCKm mutant 6 were equally virulent in slow-killing assays of C. elegans. A total of 60 to 80 worms were seeded onto NG agar inoculated with 20 μl of an overnight culture of B. pseudomallei. About 80 and 100% of the worms were killed after 24 and 72 h, respectively. E. coli OP50 was used as a negative control in this assay.
Virulence assay with C. elegans.
The KHWΔfliCKm mutants were tested to determine their abilities to kill C. elegans. On NG agar, wild-type KHW killed more than 80% of the worms after 1 day and more than 90% of the worms after 3 days (Fig. 3B). The graph for killing of C. elegans by KHWΔfliCKm mutant 6 was similar to the graph for killing by strain KHW (Fig. 3B). Thus, the KHWΔfliCKm mutant did not show any attenuation of virulence with respect to contact-dependent killing of C. elegans. The E. coli OP50 strain was used as a negative control in this assay.
Virulence assays with BALB/c mice.
In order to ascertain whether the flagellum is an essential virulence factor in an intranasal mouse model of infection, we conducted LD50 assays with BALB/c mice using both the flagellated strain KHW and the aflagellate strain KHWΔfliCKm mutant 6. The 10-day LD50 for mice intranasally infected with KHW was previously shown to be 45 CFU (15). However, the LD50 of the KHWΔfliCKm mutant was more than 8,500 CFU, the highest dose of bacteria used. Since the flagellum was shown to be not important in the pathogenesis of the bacteria during intraperitoneal infection (5), we determined the LD50 of the KHWΔfliCKm mutant in intraperitoneal infections in BALB/c mice. We found that the LD50 was more than 13,500 CFU, the highest dose used in this set of experiments, while the LD50 of KHW was 5,650 CFU. Regardless of the route of infection, all animals infected with KHWΔfliCKm remained healthy and did not succumb to disease.
We next determined if virulence could be restored through gene complementation by intranasally infecting each group of mice with 100 CFU each of KHW, KHWΔfliCKm, and the complemented mutant, KHWΔfliCKm/pUCP28TfliC. Since the kinetics of bacterial spread as measured by bacterial loads in the organs was known for KHW (15), we reasoned that a comparison with the mutant and the complemented mutant could determine if the pathogenesis of the complemented mutant was similar to that of the wild type and could shed light on the reason that the fliC mutant is unable to kill mice. Three mice from each group were sacrificed at specific times to determine the bacterial loads in the lungs and spleens. Our results show that there were very few bacteria, if any, in the KHWΔfliCKm-infected mice at all times (Table 2). In contrast, the bacterial loads in the organs were similar for KHW and the complemented mutant, KHWΔfliCKm/pUCP28TfliC (Table 2). By day 8, all four remaining mice infected with KHW were dead. Two mice infected with the complemented mutant died on day 8, and another two died on day 9, which left only one mouse for analysis on day 14. The animals had abscesses in the lungs and particularly the spleens on days 8 and 14. These results contrast sharply with those obtained in the worm assay and demonstrate that the flagellum is an important virulence determinant of B. pseudomallei in intranasally infected mice, in which the organism must move from the site of infection in the nasal cavity to the lungs.
TABLE 2.
Determination of bacterial loads in infected with KHW, KHWΔfliCKm, and KHWΔfliCKm /pUCP28TfliCa
| Strain | Organ | Expt | Bacterial load (CFU/organ)
|
|||
|---|---|---|---|---|---|---|
| 2 days after infection | 4 days after infection | 8 days after infection | 14 days after infection | |||
| KHW | Lung | 1 | NTb | 5.5 × 107 | —c | — |
| 2 | NT | 1.4 × 106 | — | — | ||
| 3 | NT | 1.4 × 104 | — | — | ||
| Spleen | 1 | NT | 1.9 × 106 | — | — | |
| 2 | NT | 4.2 × 105 | — | — | ||
| 3 | NT | 4.2 × 104 | — | — | ||
| KHWΔfliCKm | Lung | 1 | 60 | 0 | 0 | NT |
| 2 | 110 | 0 | 0 | NT | ||
| 3 | 0 | 0 | 0 | NT | ||
| Spleen | 1 | 0 | 0 | 0 | NT | |
| 2 | 0 | 0 | 0 | NT | ||
| 3 | 0 | 0 | 0 | NT | ||
| KHWΔfliCKm/pUCP28TfliC | Lung | 1 | 0 | 4.1 × 106 | 2.3 × 103 | 2.2 × 106 |
| 2 | 1.2 × 104 | 1.1 × 104 | 2.9 × 105 | — | ||
| 3 | 5.9 × 104 | 5.2 × 106 | — | — | ||
| Spleen | 1 | 3.7 × 104 | 1.9 × 106 | 4.8 × 106 | 5.0 × 107 | |
| 2 | 3.1 × 104 | 5.4 × 105 | 1.4 × 107 | — | ||
| 3 | 4.9 × 103 | 0 | — | — | ||
Ten mice were each intranasally infected with 100 CFU of KHW or KHWΔfliCKm, and 13 mice were infected with 100 CFU of KHWΔfliCKm/pUCP28TfliC.
NT, not tested.
—, no animals were available for analysis due to death from the disease.
DISCUSSION
We constructed an isogenic B. pseudomallei KHW fliC gene knockout mutant by gene replacement mutagenesis. The fliC mutant had an aflagellate and nonmotile phenotype and was unable to synthesize any flagellin. In addition, the gene replacement mutation was confirmed by PCR. RNA analysis of the expression of an adjacent downstream fliD gene showed that the mutation did not have a polar effect on the expression of downstream genes. The ability to complement the mutation by introducing a plasmid expressing wild-type B. pseudomallei fliC confirmed that the phenotype of the KHWΔfliCKm mutant was not due to a second-site mutation that might have occurred during its construction.
Flagella and motility appeared not to be essential for cell invasion by B. pseudomallei. In the cell invasion assays, the bacteria were brought into direct contact with the cells either through sedimentation by gravity during prolonged incubation or by use of a brief centrifugation step to facilitate physical contact between the bacteria and the adherent cells in the tissue culture wells. Thus, in such assays, bacterial motility was expected to be less essential in mediating the initial contact that is essential between pathogen and host cells. In contrast, the single polar flagellum in Campylobacter spp. is an important virulence determinant, and cell invasion is dependent on expression of flaA, which codes for one of the two flagellins in this organism. The nonflagellated flaA flaB and flaA flaB+ mutants invaded cultured cells at levels that were 0.06 to 1% of the levels of invasion by the wild type when the bacteria were not centrifuged to bring them into contact with the monolayer. If a centrifugation step was added, the level of invasion by the mutants increased approximately 30-fold, but it remained significantly lower than the level of invasion by the wild type (25, 26). Although it was previously shown that B. pseudomallei could enter A549 cells, there was no information about its ability to replicate in these cells (12). We showed that B. pseudomallei can also replicate intracellularly in nonphagocytic A549 cells, which demonstrates that B. pseudomallei is capable of invasion and intracellular multiplication within nonphagocytic cells and supports the notion that B. pseudomallei is adapted to an intracellular niche.
C. elegans has been used as a model genetic host to study bacterial pathogenesis. In the P. aeruginosa-mediated killing of C. elegans, contact-dependent slow killing was observed when the bacteria were grown on a low-nutrient agar, such as NG agar, whereas contact-independent fast killing was observed when the bacteria were cultured on high-osmolarity media. We have used the slow-killing assay to screen for virulence-attenuated transposon mutants of B. pseudomallei (9). The slow-killing kinetics of the flagellated B. pseudomallei strain KHW and the nonflagellated mutants with C. elegans did not differ. A similar result was obtained by O'Quinn et al., who used a fliC knockout mutant of B. pseudomallei (17). This phenomenon could be explained by direct physical contact between the pathogen and the worms in such assays, which is independent of bacterial motility. In addition, during feeding on bacteria, C. elegans uses its pharyngeal pumping mechanism to bring the bacteria into the gut, so that bacterial motility is not required for the pathogen to be brought into contact with host cells. However, the results of the virulence assay with mice showed that the aflagellate B. pseudomallei KHWfliCKm mutant is avirulent compared with the flagellated wild-type strain KHW (Table 2). Thus, our data showed that virulence-attenuated bacterial mutants which are defective in motility are likely to remain undetected in C. elegans-pathogen virulence screening analyses.
Based on our mouse model, we found that the flagellum is an important virulence determinant in the pathogenesis of B. pseudomallei. DeShazer et al. previously showed that there was not a significant difference between the virulence of the wild type and the virulence of the Tn5-OT182 disrupted fliC mutant when the pathogens were injected intraperitoneally into young diabetic rats and Syrian hamsters (5). Our results showed that the KHWΔfliCKm mutant was attenuated for virulence when mice were infected intraperitoneally with 13,500 CFU, the highest dose used in our experiments. The difference in the findings could have been due to the use of different animal models since the disease in diabetic rats and Syrian hamsters was more acute than the disease in mice. This could mean that the toxicity and faster kinetics of death, particularly in the Syrian hamster model, were due in part to the action of factors other than bacterial infection of the cells per se, such as endotoxin or soluble toxins. Our results obtained with the mouse model agree with those of Feldman et al. (8), who examined the role of flagella in the initial stages of respiratory tract infection by P. aeruginosa. These authors observed no mortality in the absence of FliC, compared to 30% mortality when the mice were intranasally infected with the wild-type strain. Since the infections ascribed to the fliC mutants were also very focal in their distribution, it was concluded that functional flagella are important for providing a means for the organism to spread throughout the respiratory tract (8). This is probably mediated through the motility function. Another function of flagella that facilitates infection is their role as an adhesin. In P. aeruginosa, FliC was shown to be an adhesin which is responsible for the binding of bacteria to Muc1, a mucin secreted by respiratory epithelial cells (14). Thus, the attenuated virulence of our fliC mutant during intranasal infection of mice could have been due to a loss of motility or adhesion or both. During intraperitoneal infection of mice, flagella could play a role in facilitating the uptake of bacteria by mononuclear cells and polymorphonuclear cells (PMNs) present in the peritoneal cavity. Several groups of workers have shown that B. pseudomallei can survive and replicate intracellularly in PMNs and monocytes without being killed (6, 13). We postulate that the uptake of bacteria by cells in the peritoneal cavity facilitates intracellular replication and spread and that this process is attenuated in the fliC mutant. Thus, another possibility to explain the results observed for the intraperitoneal infection of diabetic rats and Syrian hamsters is that flagella may not help in uptake of bacteria due to species-specific differences in the cellular receptors capable of binding flagella in these models. DeShazer et al. demonstrated previously that polyclonal antiflagellin antisera passively protected diabetic rats from intraperitoneal challenge with B. pseudomallei, which contradicted their results obtained with the fliC mutant (1). It is possible that the antisera interfered with the ability of the bacteria to replicate or that opsonized bacteria could trigger a respiratory burst in the PMNs and mononuclear cells, leading to increased microbicidal activity.
KHW and the KHWΔfliCKm mutant were equally invasive and replication proficient in the A549 lung epithelial cells. Thus, flagella are not essential for entry of B. pseudomallei into the lung epithelial cells during in vitro assays. In B. cepacia, flagellum-mediated motility appears to be required for optimal invasion of A549 cells during establishment of contact with the host cells and for bacterial entry once contact has been established (23). It has been suggested that B. cepacia might use motility to penetrate the viscous mucus layer in cystic fibrosis lung airways in order to establish contact with the underlying epithelial tissues. For Clostridium difficile, there appears to be a receptor specific for flagella in the mucus of murine intestines that facilitates colonization and establishment of an infection in the gut (22). A similar process would explain the role of the B. pseudomallei flagellin during intranasal infection but not its role during an in vitro cell invasion assay as the cell surface properties in the latter case would not be identical to those in vivo. Thus, the KHWΔfliCKm mutant, which is defective in FliC synthesis, aflagellate, and nonmotile, would be defective in establishing an intranasal infection in mice.
Acknowledgments
We thank Y. M. Ong and S. C. Lim for technical assistance and E. H. Yap and Joseph Thong for use of the containment facility in the Department of Microbiology for the work with mice.
This work was supported by grant NMRC/0285/1998 from the National Medical Research Council of Singapore and by grant R-183-000-030 from the Academic Research Fund of the National University of Singapore.
Editor: V. J. DiRita
REFERENCES
- 1.Brett, P. J., D. C. Mah, and D. E. Woods. 1994. Isolation and characterization of Pseudomonas pseudomallei flagellin proteins. Infect. Immun. 62:1914-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brett, P. J., and D. E. Woods. 2000. Pathogenesis of and immunity to melioidosis. Acta Trop. 74:201-210. [DOI] [PubMed] [Google Scholar]
- 3.Carsiotis, M., D. L. Weinstein, H. Karch, I. A. Holder, and A. D. O'Brien. 1984. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect. Immun. 46:814-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeShazer, D., P. J. Brett, R. Carlyon, and D. E. Woods. 1997. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J. Bacteriol. 179:2116-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan, A. M., and D. L. Gordon. 1996. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect. Immun. 64:4952-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 8.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan, Y. H., K. L. Chua, H. H. Chua, B. Liu, C. S. Hii, H. L. Chong, and P. Tan. 2002. Characterization of Burkholderia pseudomallei infection and identification of novel virulence factors using a Caenorhabditis elegans host system. Mol. Microbiol. 44:1185-1197. [DOI] [PubMed] [Google Scholar]
- 10.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail, G., N. Razak, R. Mohamed, N. Embi, and O. Omar. 1988. Resistance of Pseudomonas pseudomallei to normal human serum bactericidal action. Microbiol. Immunol. 32:645-652. [DOI] [PubMed] [Google Scholar]
- 12.Jones, A. L., T. J. Beveridge, and D. E. Woods. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kespichayawattana, W., S. Rattanachetkul, T. Wanun, P. Utaisincharoen, and S. Sirisinha. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect. Immun. 68:5377-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillehoj, E. P., B. T. Kim, and K. C. Kim. 2002. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am. J. Physiol. Lung Cell. Mol. Physiol. 282:L751-L756. [DOI] [PubMed]
- 15.Liu, B., G. C. Koo, E. H. Yap, K. L. Chua, and Y. H. Gan. 2002. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect. Immun. 70:504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mobley, H. L., R. Belas, V. Lockatell, G. Chippendale, A. L. Trifillis, D. E. Johnson, and J. W. Warren. 1996. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 64:5332-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Quinn, A. L., E. M. Wiegand, and J. A. Jeddeloh. 2001. Burkholderia pseudomallei kills the nematode Caenorhabditis elegans using an endotoxin-mediated paralysis. Cell Microbiol. 3:381-393. [DOI] [PubMed] [Google Scholar]
- 18.Ormonde, P., P. Horstedt, R. O'Toole, and D. L. Milton. 2000. Role of motility in adherence to and invasion of a fish cell line by Vibrio anguillarum. J. Bacteriol. 182:2326-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Simon, R., U. Priefer, and A. Puhler. 1983. Broad host range mobilization system for transposon mutagenesis of Gram negatives. Bio/Technology 1:784-791. [Google Scholar]
- 22.Tasteyre, A., M. C. Barc, A. Collignon, H. Boureau, and T. Karjalainen. 2001. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 69:7937-7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomich, M., C. A. Herfst, J. W. Golden, and C. D. Mohr. 2002. Role of flagella in host cell invasion by Burkholderia cepacia. Infect. Immun. 70:1799-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed]
- 25.Wassenaar, T. M., N. M. Bleumink-Pluym, and B. A. van der Zeijst. 1991. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 10:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassenaar, T. M., B. A. van der Zeijst, R. Ayling, and D. G. Newell. 1993. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 139:1171-1175. [DOI] [PubMed] [Google Scholar]
- 27.West, S. E., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]
- 28.Yao, R., D. H. Burr, P. Doig, T. J. Trust, H. Niu, and P. Guerry. 1994. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol. Microbiol. 14:883-893. [DOI] [PubMed] [Google Scholar]