Abstract
Enteric Salmonella infection is accompanied by inflammation and diarrhea, and yet little is known about its effects on intestinal epithelial physiology. Since species differences limit the utility of animal tissues and cell lines lack relevant cell-cell interactions, we have used a human model of fetal intestine grown as xenografts in SCID mice. We investigated here the effects of Salmonella enterica serovar Typhimurium SL1344 on xenograft ion transport. Harvested xenografts were stripped of seromuscular layers by blunt dissection, infected with Salmonella, and mounted in Ussing chambers. Salmonella infection for 1 h increased baseline ion transport without altering tissue conductance or morphology. The increased transport was blocked by the cyclooxygenase inhibitor, indomethacin, or the specific Cox-2 inhibitor, NS-398. Further, xenografts infected for 2 h showed increased secretory responses to the calcium-dependent agonist, carbachol, and the cyclic AMP-dependent agonists prostaglandin E2 (PGE2) and forskolin, which were blocked by indomethacin. Western blot experiments revealed that infection was accompanied by increased cyclooxygenase 2 (Cox-2) expression, with no change in Cox-1 levels. Immunoassay demonstrated basolateral PGE2 release, which was inhibited by indomethacin. Histological examination of infected xenografts illustrated that upregulated Cox-2 expression was restricted to the epithelium and that little or no invasion of the tissue by Salmonella occurred for up to 2 h. In summary, Salmonella infection rapidly increases Cox-2 expression in human intestinal tissue, accounting for increased epithelial ion transport characteristic of infectious diarrhea.
The hallmark of a range of intestinal infectious diseases is diarrhea. With the exception of intestinal pathogens that elaborate enterotoxins, the mechanisms underlying intestinal dysfunction during infections with many bacteria remain largely unestablished. The intestinal epithelium is the initial site of contact between enteric microorganisms and the gastrointestinal mucosa and is a continuous layer of cells lining the entire gastrointestinal tract. It acts as a physical barrier, preventing the entry of harmful substances from the intestine into the body, and transports water to and from the intestinal contents. Water transport is a passive process, which is driven by the active transport of ions and nutrients. Normally, there is a net absorption of ions and water but in conditions of disease, such as intestinal bacterial infection, the epithelium can rapidly convert from its absorptive to a net secretory phenotype.
Salmonellae are gram-negative bacteria that can cause gastroenteritis and enteric fever and represent a major public health problem worldwide. All Salmonella serotypes share the ability to invade their host by inducing their own uptake into nonphagocytic cells of the intestinal epithelium. They facilitate this uptake through proteins secreted by a type III protein secretion system encoded within the Salmonella pathogenicity island 1 that also encodes the effector proteins required for invasion (13). This system is abundant in several bacteria and, in addition to being responsible for invasion, functions to secrete virulence proteins into host cells. Thus, through secreted virulence proteins, through invasion, or both, Salmonella can induce changes in the epithelium, altering the function of the intestinal lining and presumably converting it to a hypersecretory state. However, the underlying mechanism(s) by which Salmonella induces diarrhea are not fully understood.
The understanding of diarrheal disease requires the application of model systems. Animal tissues are commonly used but have a number of shortcomings due to significant species differences between experimental animals and human hosts. Moreover, studies with isolated epithelial cell lines lack relevant cell-cell interactions. Thus, new models for studying intestinal bacterial infections are being sought. In this regard, we have previously described a human-derived tissue model for the study of ion transport mechanisms (4). This model employs fetal intestinal tissues grown as xenografts in severe combined immunodeficient (SCID) mice (22). The main advantage of intestinal xenografts is that they provide a model consistent with intact human intestinal tissue, which allows for prolonged infection with bacteria and enables studies of the interactions of different cell types, in both human small and large intestine, during bacterial infection.
Infection studies with epithelial cell lines have shown that Salmonella induces host cell responses, including chemokine and cytokine secretion (8, 9), and upregulation of expression of cyclooxygenase 2 (Cox-2) (8, 21). Coculture studies also show transepithelial migration of polymorphonuclear leukocytes early after Salmonella-epithelial cell contact (17) and that epithelial invasion by Salmonella is not necessary for polymorphonuclear leukocyte movement (11). However, changes in the secretory phenotype of intestinal epithelial cell lines show a considerable latency after Salmonella infection and are difficult to dissociate from frankly injurious effects of the infectious process (21). These various observations suggest that interactions between intestinal epithelial cells and Salmonella play a key role in orchestrating the inflammatory response (9, 17, 18) and underscore the limitations inherent in cell line models to understand the full picture of Salmonella infection in human tissue. It is therefore relevant to the elucidation of Salmonella pathogenesis to utilize the intestinal xenograft model to extend findings obtained with cell lines to a more physiological setting, in which cell-cell interactions may amplify responses to the pathogen. Thus, we sought to examine here whether Salmonella infection of xenografts had any consequences in terms of transport function and the characteristics and mechanisms of any responses seen.
(This work was presented in part at the 2001 meeting of the American Gastroenterological Association and has been published in abstract form [Gastroenterology 120:A3812, 2001].)
MATERIALS AND METHODS
Materials.
Blocking reagent for Western blotting (skim milk), Rainbow colored protein molecular weight markers, ECL Plus, and PGE2 enzyme immunoassay were obtained from Amersham Pharmacia Biotech, Piscataway, N.J. BM chemiluminescence enzyme-linked immunosorbent assay reagent was from Boehringer Mannheim, Mannheim, Germany, and NS-398 and indomethacin were from Cayman Chemical, Ann Arbor, Mich. Carbachol (CCh), PGE2, and forskolin were obtained from Sigma-Aldrich, St. Louis, Mo., and anti-Cox-1 (H-62; rabbit polyclonal immunoglobulin G [IgG]) and anti-Cox-2 (N-20) antibodies (goat polyclonal IgG) were from Santa Cruz Biotechnology, Inc., Santa Cruz, Calif. Hoechst 33258 was purchased from Molecular Probes, Inc., Eugene, Oreg. All other chemicals were of at least reagent grade and were obtained commercially. Serovar Typhimurium SL1344 was generously provided by Donald G. Guiney of University of California, San Diego.
Human intestinal xenografts.
Segments of human fetal small intestine (gestational age, 16 to 20 weeks) were transplanted subcutaneously into the rear flanks of SCID mice as previously described (22). At times greater than 10 weeks thereafter, the intestinal xenografts resembled morphologically mature pediatric intestine, with an epithelium of entirely human origin. Experimental procedures were approved by the UCSD Human and Animal Subjects Committees.
Measurement of ion transport across human intestinal xenografts.
Salmonella were maintained by using Luria-Bertani (LB) broth or LB agar plates. Prior to infection, Salmonella strains were grown overnight without shaking in LB broth supplemented with 300 mM NaCl. After they reached an optical density at 600 nm of ca. 0.4, the bacteria were washed in phosphate-buffered solution (PBS) and resuspended in Ringer's solution. Xenografts were allowed to mature for at least 10 weeks, and then they were harvested, stripped of seromuscular layers by blunt dissection, mounted horizontally in chambers, and infected from the mucosal aspect with serovar Typhimurium SL1344 with ca. 5 × 106 bacteria/0.25-cm2 tissue (or incubated with buffer for controls) for various times. Stripped xenografts were then mounted vertically in Ussing chambers (aperture = 0.07 cm2). The mucosal sheets were bathed in circulating Ringer's solution composed of 141.0 mM Na+, 4.2 mM K+, 3.0 mM Ca2+, 1.2 mM Mg2+, 125.8 mM Cl−, 27.6 mM HCO3−, and 3.2 mM H2PO42−, with 10 glucose (serosal side) or 10 mannitol (mucosal side) and gassed with 95% O2 and 5% CO2. The tissues were voltage clamped to zero potential difference by the continuous application of short-circuit current (Isc). Changes in ion transport across the mucosa in response to agonists were recorded either as changes in Isc (ΔIsc) required to maintain a zero potential difference (PD) or as the open circuit PD measured at 15-s intervals. At all other times, short circuiting was maintained to negate any transepithelial electrochemical driving force.
Immunoprecipitation and Western blotting.
Stripped xenografts were infected mucosally with S. enterica serovar Typhimurium SL1344 as described above. The tissue was then placed in ice-cold lysis buffer (consisting of 20 mM Tris base [pH 7.4], 20 mM NaCl, 10 mM MgCl2 · 6H2O, 1 mM NaVO4, 1 μg of leupeptin/ml, 100 μg of phenylmethylsulfonyl fluoride/ml, and 50 μg of gentamicin/ml), homogenized, and incubated at 4°C for 30 min. The samples were then centrifuged at 10,000 rpm for 10 min, and the pellet was discarded. An aliquot was removed from each sample to determine the protein content, and samples were adjusted so that they contained equal amounts of protein. The samples were boiled for 5 min, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Separated proteins were transferred onto polyvinylidene difluoride membranes (DuPont NEN) overnight at 4°C. The membranes were washed in 1% blocking buffer for 30 min, followed by incubation of the membrane with the appropriate dilution of primary antibody in 1% blocking buffer for 60 min. This was followed by three washes in Tris-buffered saline with 1% Tween (TBST). After the washes, horseradish peroxidase-conjugated secondary antibodies were added to the membrane in 1% blocking buffer and allowed to incubate for an additional 30 min. This was followed by three further washes in TBST. Immunoreactive proteins were detected by using an enhanced chemiluminescence detection kit and exposure of the membrane to X-ray film. Quantitation of protein bands was performed by densitometry using NIH Image software.
Histological analysis of xenografts.
For immunohistochemical analysis, infected, and control intestinal xenografts were embedded in OCT compound (TissueTek; Miles Scientific, Naperville, Ill.) and either snap-frozen in methylbutene-dry ice or fixed in 10% neutral buffered formalin. Formalin-fixed tissues were embedded in paraffin, and 5-μm sections were prepared. Immunostaining was visualized with Cy3-conjugated rabbit anti-mouse or donkey anti-goat antibodies. From snap-frozen tissue samples, serial cryostat sections (5 μm) were prepared, fixed in 10% formalin for 10 min, and blocked (1% bovine serum albumin, 0.2% blocking reagent, 0.3% Triton-X-100). Sections were incubated with the respective antibodies, subsequently washed three times in phosphate-buffered saline, and then incubated with donkey anti-goat IgG GY3- or anti-mouse IgG fluorescein isothiocyanate-conjugated secondary antibody (Amersham Pharmacia Biotech). Nuclei were visualized by staining with Hoechst 33258 dye.
PGE2 enzyme immunoassay.
Xenografts stripped of seromuscular layers were placed in horizontal chambers and infected with Salmonella organisms as described above with or without indomethacin (10 μM) in a solution containing 10 μM arachidonic acid. Control tissues were studied in parallel. After 2 h of infection, the apical and basolateral bathing solutions were collected and analyzed for secreted PGE2 by a commercially available PGE2 enzyme immunoassay (Amersham Pharmacia Biotech) in accordance with the manufacturer's protocol.
Statistical analysis.
All results are expressed as the mean ± the standard error of the mean (SEM) for a series of experiments. Student t tests were used to compare paired data. One-way analysis of variance with a Student Neuman-Keuls post-test was used when three or more groups of data were compared. P values of <0.05 were considered significant.
RESULTS
Examination of xenograft invasion by S. enterica serovar Typhimurium.
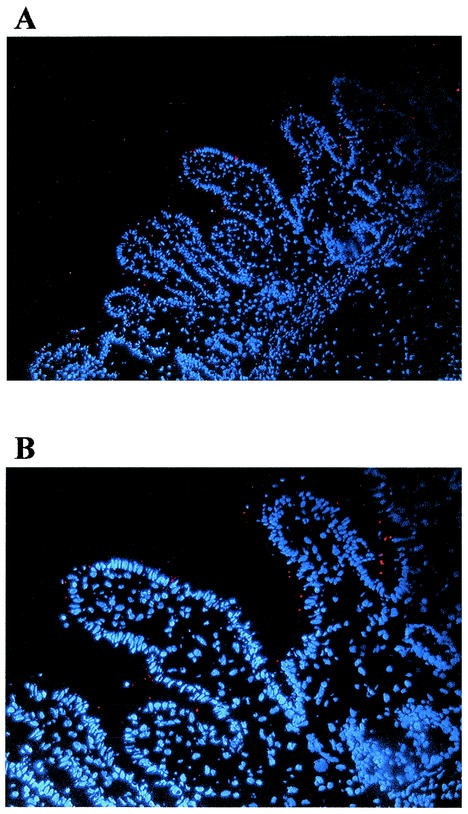
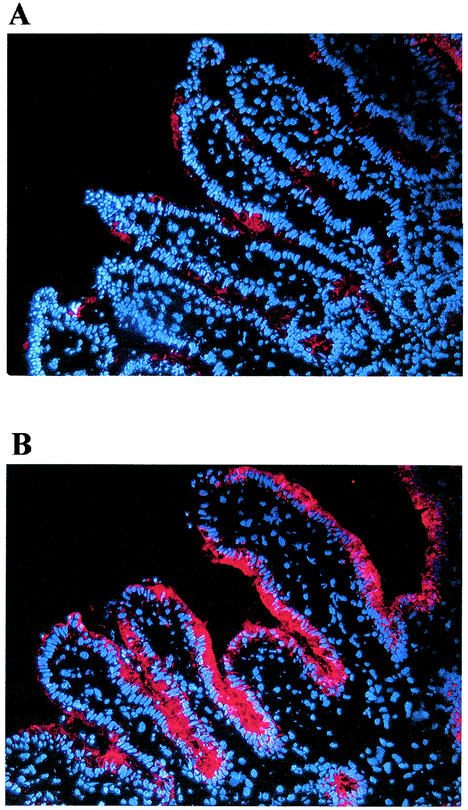
Salmonellae are highly invasive for intestinal epithelial cells (8, 9, 10, 20). To determine Salmonella infection in intact human intestinal tissue, we infected the xenografts with serovar Typhimurium and examined them by histology. At selected times after infection, tissues were snap-frozen and stained for Salmonella (red). Histological examination of infected xenografts showed little or no appreciable invasion of the tissue by serovar Typhimurium at up to 2 h (Fig. 1). However, costaining for Salmonella and F-actin showed a close association between Salmonella and the epithelium (data not shown). In control experiments, we used CFU measurements to confirm that the serovar Typhimurium strain used was able to invade T84 intestinal epithelial cells after a 1-h incubation period (data not shown).
FIG. 1.
Salmonella infection in human intestinal xenografts. Human intestinal xenografts were infected with serovar Typhimurium SL1344 for 2 h, embedded in OCT compund, and snap-frozen in methylbutene-dry ice. Histological analysis of Salmonella-infected xenografts showed attachment but no significant Salmonella (red) invasion of the tissue at up to 2 h of infection. (A) Low power view; (B) a higher magnification of the section shown in panel A.
Serovar Typhimurium infection increased baseline ion transport, which could be inhibited by indomethacin and NS-398.
Despite the unexpected finding that serovar Typhimurium was not found in deeper layers of the tissue at the times studied, we were interested in determining any physiological effects that might appear. Salmonella infection can cause diarrhea within a short time (hours) after ingestion, and thus any rapid effects of the pathogen on intact human tissues would be clinically relevant.
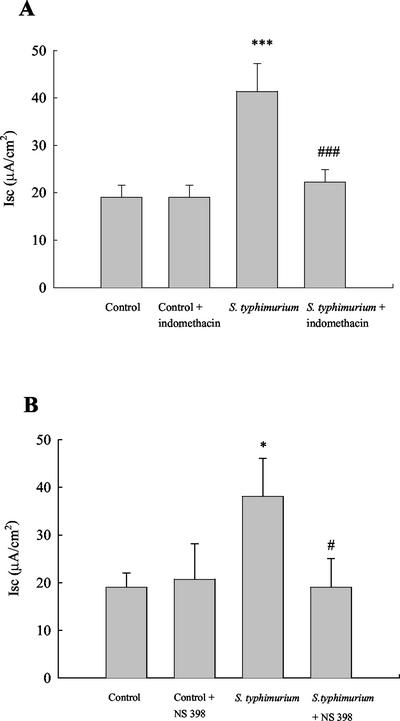
We first examined whether ex vivo infection with serovar Typhimurium alters basal transport properties of human intestinal xenografts. As shown in Fig. 2A, serovar Typhimurium infection significantly increased baseline ion transport compared to controls (38.1 ± 3.6 versus 14.3 ± 5.6 μA/cm2, n = 6, P < 0.01) at 1 h of infection. This was not accompanied by any differences in conductance (160 ± 47 mS/cm2 [control] versus 163 ± 29 mS/cm2 [infected]). Moreover, infection of xenografts in the presence of indomethacin (10 μM) reversed the increase in baseline secretion by 88.9% ± 19.3% (n = 9), implicating cyclooxygenase activity in the response. To elucidate whether the effect was dependent on the Cox-2 isoform of cyclooxygenase, we incubated the tissue with the Cox-2 specific inhibitor, NS-398 (3 μM). This drug was also able to abolish the increase in ion transport in infected tissue by 99.9% ± 38.9% (n = 7; Fig. 2B). Neither inhibitor had any effect on basal ion transport in uninfected tissue. Thus, the ability of Salmonella to enhance transport responses might be secondary to the generation of products of Cox-2 activity.
FIG. 2.
Effect of Salmonella infection on basal ion transport. Intestinal xenografts stripped of their seromuscular layers were infected with serovar Typhimurium for 1 h prior to mounting in modified Ussing chambers. Salmonella infection increased baseline ion transport in human intestinal xenografts. (A) The cyclooxygenase inhibitor indomethacin (10 μM) did not alter baseline ion transport in control cells but did inhibit the enhanced transport induced by Salmonella in infected tissue. ✽✽✽, P < 0.001 versus control; ###, P < 0.001 versus responses in infected tissue (n = 9). (B) Similarly, the specific inhibitor of Cox-2, NS-398 (3 μM), did not alter baseline ion transport in control tissue but reversed the increase in baseline secretion observed in Salmonella-infected tissue. ✽, P < 0.05 versus control; #, P < 0.05 versus Salmonella-infected tissue (n = 7).
Ca2+- and cAMP-induced ion transport was increased in Salmonella-infected xenografts compared to controls.
Many agonists can stimulate chloride secretion, although most utilize common secretory machinery and one of two main signaling pathways induced either by cyclic nucleotides, such as cyclic AMP (cAMP), or by intracellular Ca2+. Increases in cytosolic cAMP induce sustained chloride secretion via activation of cystic fibrosis transmembrane regulator chloride channels, whereas increases in Ca2+ induce smaller and transient chloride secretory responses (3). It was of interest, therefore, to examine whether infection with serovar Typhimurium had any effect on transport responses stimulated by either cAMP- and Ca2+-dependent agonists.
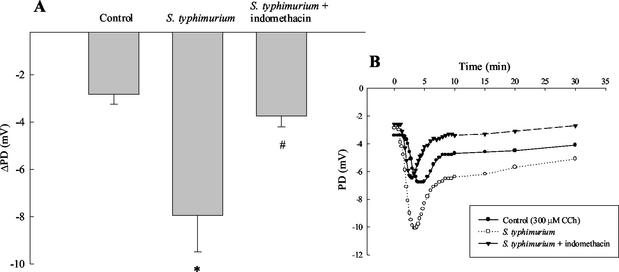
When xenografts were stimulated with the Ca2+-dependent agonist, CCh, ion transport was significantly increased after 2 h of serovar Typhimurium infection compared to controls (Fig. 3A). Note that in these studies we used open circuit PD to follow transport responses rather than Isc to more accurately quantitate transport in these small tissue specimens. Changes in PD are expressed as the incremental change after agonist addition (ΔPD). The figure inset shows a typical time course for the absolute PD responses to CCh. It has been known for some time that chloride secretory responses to CCh can be synergistically enhanced by cAMP-dependent agonists, including prostaglandins, as studied in intestinal epithelial cell lines (2, 6, 24). Thus, the increased secretory response to CCh in the infected xenograft could result from a similar synergistic interaction. In control experiments, xenografts were infected either with serovar Typhimurium or with the nonpathogenic Escherichia coli strain, DH5α. In these experiments, ion transport responses induced by CCh were increased by 417 ± 163% in tissues infected with Salmonella and 143% ± 49% in tissues infected with E. coli DH5α (n = 9). These data suggest that the ability of the pathogen to increase secretagogue-stimulated ion transport was not a general property common to all bacteria. E. coli DH5α also failed to significantly alter basal ion transport.
FIG. 3.
Increased CCh-stimulated ion transport responses in infected xenografts are reversed by indomethacin. Xenografts were stripped of their seromuscular layers, infected with Salmonella with or without concurrent treatment with the cyclooxygenase inhibitor indomethacin (10 μM) for 1 h, mounted in Ussing chambers, and allowed to equilibrate for 1 h prior to stimulation with agonists. (A) When xenografts were stimulated with the Ca2+-dependent agonist, CCh, ion transport, measured as a decrease in PD, was significantly increased after 2 h of serovar Typhimurium infection compared to controls. ✽, P < 0.05 versus control (n = 5). In tissues that were treated with indomethacin, the Salmonella-induced enhanced secretory responses to CCh were inhibited. #, P < 0.05 versus Salmonella-infected tissue (n = 5). The data shown in panel A represent the incremental changes in PD evoked by CCh addition (i.e., ΔPD). (B) Typical time course of absolute PD response to CCh under the three conditions studied.
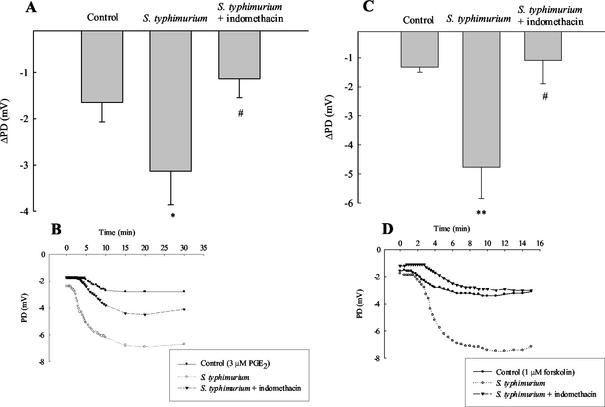
Similar to the data in Fig. 3A, when xenografts were stimulated with the cAMP-dependent agonist PGE2, ion transport was significantly increased after 2 h of serovar Typhimurium infection compared to controls (Fig. 3B). The figure inset shows a typical time course of the PD response to PGE2. In some ways this was surprising, since if infection induces the production of endogenous prostanoids, one might predict that the tissue could become desensitized to exogenous addition of such secretagogues. We therefore examined whether infection also increased responses to another cAMP-dependent secretagogue, forskolin. In fact, a significantly increased PD response to forskolin was seen in tissues infected with serovar Typhimurium for 2 h compared to controls (−2.7 ± 0.5 mV versus −1.5 ± 0.3 mV, P < 0.05, n = 9). Similar control experiments as mentioned above for CCh were also carried out for forskolin. Xenografts were infected with either serovar Typhimurium SL 1344 or nonpathogenic E. coli DH5α. Secretory responses to forskolin were increased by 381 ± 203% and 113% ± 27% for Salmonella- and E. coli-infected xenografts, respectively (n = 7), again suggesting that enhanced secretory responses are specific for pathogenic bacterial infection of the tissue.
Indomethacin diminished the increased agonist-evoked ion transport due to infection.
We next examined whether enhanced transport responses to secretagogues were related to infection-induced changes in prostanoid synthesis, as seen for the increase in basal ion transport. The cyclooxygenase inhibitor, indomethacin (10 μM), reversed the increase in ion transport responses to CCh (300 μM) seen in xenografts infected with serovar Typhimurium. As shown in Fig. 3A, this drug essentially abolished the effect of infection on responsiveness to CCh when transport responses were expressed as the change in PD from baseline. Again, Fig. 3B shows typical time courses of PD responses to CCh under the three conditions studied. Similar results were obtained in Salmonella-infected tissues stimulated with PGE2. The presence of indomethacin also abolished the enhanced transport responses to PGE2 seen in infected tissue (Fig. 4A and B). Finally, similar to the data in Fig. 4A and B, indomethacin (10 μM) also reversed enhanced ion transport responses stimulated by forskolin (1 μM) in serovar Typhimurium-infected xenografts (Fig. 4C and D).
FIG. 4.
Increased cAMP-dependent ion transport responses in infected xenografts are reversed by indomethacin. Stripped xenograft tissues were infected with Salmonella with or without concurrent treatment with the cyclooxygenase inhibitor indomethacin (10 μM) for 1 h, mounted in Ussing chambers, and stabilized for 1 h prior to stimulation with agonists. (A) In tissues that were treated with indomethacin the Salmonella-induced enhanced secretory responses to PGE2 were inhibited. The data are expressed as incremental changes in PD evoked by PGE2 addition (i.e., ΔPD). ✽, P < 0.05 versus control; #, P < 0.05 versus Salmonella-infected tissue (n = 5). (B) Typical time courses of the absolute PD responses to PGE2 under the conditions studied. (C and D) Similar results obtained with another cAMP-dependent agonist, forskolin (1 μM). ✽✽, P < 0.01 versus control; #, P < 0.05 versus Salmonella-infected tissue (n = 6).
Salmonella infection increased Cox-2 but not Cox-1 expression.
In light of our findings with indomethacin and NS-398, we suggested that the effects of serovar Typhimurium on xenograft transport responses might be due to the induction of cyclooxygenase activity. We used Western blotting approaches to examine levels of the inducible cyclooxygenase isoform, Cox-2. As shown in Fig. 5, there were no changes in Cox-2 levels in control tissues over time. However, serovar Typhimurium infection was accompanied by a progressive increase in Cox-2 expression that was statistically significant at 1 h and 2 h. This increase in Cox-2 expression was not obtained when similar experiments were performed with heat-killed serovar Typhimurium, indicating that cyclooxygenase induction requires viable bacteria (data not shown). Moreover, in contrast to Cox-2, serovar Typhimurium infection had no effect on levels of Cox-1 compared to those seen in control human intestinal xenografts (Fig. 5).
FIG. 5.
Salmonella infection induces Cox-2 but not Cox-1 expression. Stripped human intestinal xenografts were infected with Salmonella for the times indicated and tissue lysates were analyzed by Western blotting with antibodies specific for Cox-2 or Cox-1. (A) Representative example of Cox-2 staining and densitometric analysis of several similar experiments. The data are expressed as the means ± the SEM for Cox-2 levels in control tissues and tissues exposed to Salmonella infection, as measured in arbitrary units (a.u.) (n = 5). Asterisks denote significant differences from control, uninfected tissue for the respective time point: ✽✽, P < 0.01; ✽✽✽, P < 0.001. (B) The figure shows a representative Western blot and densitometric analysis of several similar experiments in which the data are expressed as the means ± the SEM (n = 3). Cox-1 expression was not altered in Salmonella-infected tissue compared to controls at any of the time points studied.
Increased Cox-2 expression occurs primarily in epithelial cells of infected xenografts.
Immunohistological examination of serovar Typhimurium-infected xenografts showed that the increase in Cox-2 expression was localized predominantly to the epithelium. As seen in Fig. 6, the control tissue constitutively expressed low levels of Cox-2, in keeping with prior observations of others (5, 14). When xenografts were infected with serovar Typhimurium, Cox-2 expression was markedly increased, and the increase appeared to be restricted largely to the epithelium, along the full length of the villus.
FIG. 6.
Salmonella-induced Cox-2 expression is restricted to the epithelium. Human intestinal xenografts infected for 2 h with Salmonella were embedded in OCT compound and snap-frozen in methylbutene-dry ice. Histological analysis of control and Salmonella-infected tissue illustrates that the induction of Cox-2 expression is restricted to the epithelium and occurred along the entire crypt-villus axis. (A) Control tissue with a low level of background staining for Cox-2. (B) Cox-2 staining in tissue infected with Salmonella for 2 h.
Salmonella infection induces basolateral PGE2 release that is sensitive to inhibition by indomethacin.
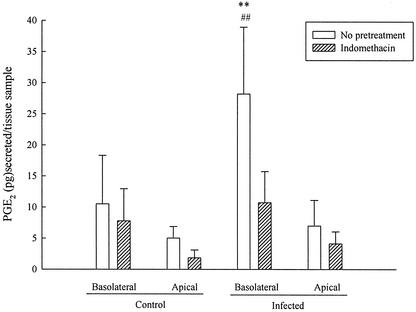
Finally, either control or infected xenografts were incubated for 2 h in the presence or absence of indomethacin, and the basolateral and apical solutions were analyzed for secreted PGE2 by an immunoassay. As illustrated in Fig. 7, when xenografts were infected with Salmonella, basolateral PGE2 secretion was significantly increased compared to control tissues, whereas apical secretion was unaltered. The enhanced basolateral secretion of PGE2 seen in infected tissues was blocked by indomethacin.
FIG. 7.
Indomethacin decreases Salmonella-induced PGE2 secretion into the basolateral medium. Xenografts stripped of seromuscular layers were infected with Salmonella for 2 h in the presence or absence of indomethacin, and apical and basolateral solutions were analyzed for secreted PGE2 by enzyme-linked immunosorbent assay. Salmonella infection induced a significant increase in basolateral PGE2 secretion, whereas apical PGE2 secretion was unaltered compared to control tissues. The enhanced PGE2 secretory responses to Salmonella were reversed in the presence of 10 μM indomethacin. ✽✽, Significantly greater than corresponding basolateral release in the presence of indomethacin (P < 0.01); ##, significantly greater than basolateral release in uninfected tissue (P < 0.01) (as determined by analysis of variance; n = 5).
DISCUSSION
The interactions that occur between pathogenic microorganisms and their host cells are complex and intimate. The studies reported here have focused on a clinically important enteric pathogen, Salmonella, which can cause gastroenteritis and enteric fever and contributes significantly to worldwide public ill health. We have shown here that the ability of Salmonella to induce upregulation of Cox-2 expression accounts, at least in part, for increased ion transport in human intestinal xenografts, perhaps paralleling the diarrheal symptoms induced by this pathogen in human subjects.
In the present study, we found that tissue invasion does not seem to be necessary for Salmonella to induce the early effects on ion transport and Cox-2 expression found here. Actual invasion of enterocytes has also been shown not to be required for other pathogenic responses to Salmonella infection, including activation of nuclear factor-κB (7), which plays a significant role in the regulation of immune and inflammatory response genes in intestinal epithelial cells (23). Further, infection of this human tissue model induces functional changes much more rapidly than observed in experiments with the same bacterial strain conducted in human cell line models.
Data from the present study further showed that serovar Typhimurium, but not a nonpathogenic E. coli strain, rapidly increases basal, as well as Ca2+- and cAMP-stimulated, ion transport in human intestinal xenografts and that Cox-2 inhibitors reversed these increases, as well as Salmonella-induced PGE2 release. This extends the findings of earlier studies by Eckmann et al. (8), who showed that S. enterica serovar Dublin and serovar Typhimurium were able to induce PGE2 and PGF2α production in intestinal epithelial cell lines. These same authors (8) also showed that conditioned media from serovar Dublin-infected HT-29 cells was capable of inducing chloride secretion by naive T84 cells, an effect attributable to the secreted prostaglandins.
Synergism is known to occur between the secretory effects of Ca2+- and cAMP-dependent agonists (2, 6, 24), and Cox-2 induction in infected tissue is observed at times consistent with the induction of increased chloride secretion. Thus, the increased responses to CCh can likely be explained as synergistic responses evoked by stimulation in the context of prostaglandins produced by Cox-2. The potentiated responses to cAMP-dependent secretagogues are, however, more difficult to explain. However, many ion channels are affected by arachidonic acid and/or the various metabolites of arachidonic acid (19), and we have recently shown that bacterial infection of HT29/cl.19 cells increases total and membrane abundance of cystic fibrosis transmembrane regulator and the Na+,K+,2Cl− cotransporter (NKCC-1) in a manner dependent on the induction of Cox-2 (21). Previous studies (12, 15, 16) have also shown that cAMP-dependent chloride secretion, such as that evoked by forskolin or PGE2, is critically dependent on the membrane abundance of at least NKCC-1 to provide for sustained chloride entry across the basolateral membrane. Thus, the rapid effects we observed on cAMP-induced secretion may be explained by an effect of Salmonella to increase NKCC-1 in the basolateral membrane and/or to increase synthesis of this transporter.
In control tissues, Cox-2 was only expressed at low levels, but levels of this enzyme increased rapidly after Salmonella infection. In contrast, Cox-1 was constitutively expressed, and Salmonella infection did not alter expression of this protein. These data suggest that tonic production of prostaglandins by Cox-1 is likely not involved in the basal regulation of transport, whereas induction of prostanoid synthesis secondary to Cox-2 expression can rapidly upregulate ion and likely fluid secretion. The effect of selective Cox-1 and -2 inhibitors on cholera toxin (CT)-induced fluid secretion has recently also been studied in infected rat jejunum (5). That study showed, similar to results reported here for Salmonella, that CT enhanced Cox-2 levels and that the increase in Cox-2 contributes to the abundant fluid secretion after CT infection (5). Similar findings were obtained in another recent study investigating Cox-2 expression in rabbit intestinal tissue after Clostridium difficile infection (1) and in enteroinvasive E. coli- and serovar Dublin-infected HT29/cl.19A cells (21). Thus, even though these data were obtained by utilizing different bacterial infections, the intestinal epithelium responded in a similar manner by increasing Cox-2 expression.
The increased Cox-2 expression in infected human intestinal xenografts is observed predominantly in the epithelium with expression levels increasing from the crypt to villus during infection and essentially no expression in the underlying tissue. An increase in Cox-2 expression after S. enterica serovar Typhi infection in human intestinal tissue has been reported previously (8), but here we have significantly extended that finding by demonstrating the functional consequences of Cox-2 induction in serovar Typhimurium-infected tissue.
In summary, our model proposes that the initial increase in secretion observed after Salmonella infection, as well as initial Salmonella-induced signaling may not be dependent on bacterial invasion. Instead, human intestinal tissue responds to infection with serovar Typhimurium by rapidly increasing Cox-2 expression, which in turns accounts for increased secretion. Further, responses obtained in intact human tissue are much faster than those observed in cell culture models, which underscore the importance of this complementary approach using a novel and more physiologic system. Our data are consistent with the hypothesis that subepithelial elements communicate with infected epithelial cells to augment their response to the bacteria. However, the detailed mechanisms and signals by which Salmonella induces these effects still need to be examined. In any event, the secretory response could be considered as a primitive, nonimmune defense mechanism allowing the intestine to “flush out” the unwanted bacteria. The unique nature of the present study derives from its ability to examine Salmonella infection in intact human intestinal tissue, thereby allowing studies of infection in a more physiological setting. From the present study, we conclude that augmented Cox-2 expression is responsible for increased epithelial ion transport in infected tissue, which likely underlies secretory diarrhea associated with Salmonella infection. A greater understanding of the specific host signaling pathways and intracellular messengers evoked by invasive bacteria might ultimately lead to development of new therapies for the treatment of infectious diarrhea.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (DK35108 Unit 5).
We thank Donald G. Guiney for supplying the bacteria used in this study and Glenda Wheeler for assistance with manuscript preparation.
Editor: B. B. Finlay
REFERENCES
- 1.Alcantara, C., W. F. Stenson, T. S. Steiner, and R. L. Guerrant. 2001. Role of inducible cyclooxygenase and prostaglandins in Clostridium difficile toxin A-induced secretion and inflammation in an animal model. J. Infec. Dis. 184:648-652. [DOI] [PubMed] [Google Scholar]
- 2.Bajnath, R. B., N. van den Berghe, H. R. De Jonge, and J. A. Groot. 1993. Activation of ion transport by combined effects of ionomycin, forskolin and phorbol ester on cultured HT-29cl.19A human colonocytes. Pfluegers Arch. 425:90-99. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, K. E., and S. J. Keely. 2000. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu. Rev. Physiol. 62:535-572. [DOI] [PubMed] [Google Scholar]
- 4.Bertelsen, L. S., L. Eckmann, S. Resta-Lenert, and K. E. Barrett. 2000. Human intestinal xenografts as a new model for ion transport studies. Gastroenterology 118:A3118. [Google Scholar]
- 5.Beubler, E., R. Schuligoi, A. K. Chopra, D. A. Ribardo, and B. A. Peskar. 2001. Cholera toxin induces prostaglandin synthesis via post-transcriptional activation of cyclooxygenase-2 in the rat jejunum. J. Pharmacol. Exp. Ther. 297:940-945. [PubMed] [Google Scholar]
- 6.Dharmsathaphorn, K., and S. J. Pandol. 1986. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J. Clin. Investig. 77:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaves-Pyles, T., C. Szabó, and A. L. Salzman. 1999. Bacterial invasion is not required for activation of NK-κB in enterocytes. Infect. Immun. 67:800-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckmann, L., W. F. Stenson, T. C. Savidge, D. C. Lowe, K. E. Barrett, J. Fierer, J. R. Smith, and M. F. Kagnoff. 1997. Role of intestinal epithelial cells in the host secretory response to infection by invasive bacteria. J. Clin. Investig. 100:296-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galàn, J. E. 1996. Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol. 20:263-271. [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz, A. T., A. M. Siber, J. L. Madara, and B. A. McCormick. 1999. Orchestration of neutrophil movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infect. Immun. 67:608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas, M., and B. Forbush III. 1998. The Na-K-Cl cotransporters. J. Bioenerg. Biomembr. 30:161-172. [DOI] [PubMed] [Google Scholar]
- 13.Hansen-Wester, I., and M. Hensel. 2001. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 3:549-559. [DOI] [PubMed] [Google Scholar]
- 14.MacNaughton, W. K., and K. Cushing. 2000. Role of constitutive cyclooxygenase-2 in prostaglandin-dependent secretion in mouse colon in vitro. J. Pharmacol. Exp. Ther. 293:539-544. [PubMed] [Google Scholar]
- 15.Matthews, J. B., I. Hassan, S. Meng, S. Y. Archer, B. J. Hrnjez, and R. A. Hodin. 1998. Na-K-2Cl cotransporter gene expression and function during enterocyte differentiation: modulation of Cl− seretory capacity by butyrate. J. Clin. Investig. 101:2072-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews, J. B., K. J. Tally, and J. A. Smidt. 1995. Activation of intestinal Na-K-2Cl cotransport by 5′-AMP requires F-actin remodeling. Am. J. Surg. 169:50-56. [DOI] [PubMed] [Google Scholar]
- 17.McCormick, B. A., C. A. Parkos, S. P. Colgan, D. K. Carnes, and J. L. Madara. 1998. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelial by Salmonella typhimurium. J. Immunol. 160:466.. [PubMed] [Google Scholar]
- 18.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meves, H. 1994. Modulation of ion channels by arachidonic acid. Prog. Neurobiol. 43:175-186. [DOI] [PubMed] [Google Scholar]
- 20.Pace, J., M. J. Hayman, and J. E. Galàn. 1993. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell 72:505-514. [DOI] [PubMed] [Google Scholar]
- 21.Resta-Lenert, S., and K. E. Barrett. 2002. Infection with enteroinvasive bacteria alters barrier function and transport properties of human intestinal epithelial cells: role of iNOS and Cox-2 expression. Gastroenterology 122:1070-1087. [DOI] [PubMed] [Google Scholar]
- 22.Savidge, T. C., A. L. Morey, D. J. Ferguson, K. A. Fleming, A. N. Shmakov, and A. D. Phillips. 1995. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation 58:361-371. [DOI] [PubMed] [Google Scholar]
- 23.Thanos, D., and T. Maniatis. 1995. NF-κB: a lesson in family values. Cell 80:529-532. [DOI] [PubMed] [Google Scholar]
- 24.Vajanaphanich, M., C. Schultz, R. Y. Tsien, A. E. Traynor-Kaplan, S. J. Pandol, and K. E. Barrett. 1995. Cross-talk between calcium and cAMP-dependent intracellular signaling pathways. J. Clin. Investig. 96:386-393. [DOI] [PMC free article] [PubMed] [Google Scholar]