Abstract
We analyzed a previously constructed stress-sensitive Streptococcus mutans mutant Tn-1 strain resulting from disruption by transposon Tn916 of a gene encoding a protein exhibiting amino acid sequence similarity to the Escherichia coli diacylglycerol kinase. It was confirmed that the mutation led to significantly reduced lipid kinase activity, while expression of the intact gene on a plasmid restored both kinase activity and the wild-type phenotype. Further analysis revealed that the product of the dgk gene in S. mutans predominantly recognizes a lipid substrate other than diacylglycerol, most likely undecaprenol, as demonstrated by its efficient phosphorylation and the resistance of the product of the reaction to saponification. The physiological role of the product of the dgk gene as a putative undecaprenol kinase was further supported by a significantly higher sensitivity of the mutant to bacitracin compared with that of the parental strain.
A family of bacterial diacylglycerol kinases (DGKs) constitutes a group of highly hydrophobic proteins exhibiting little amino acid sequence similarity to their eukaryotic counterparts. DGK from Escherichia coli, the only well-characterized member of the family, is an integral membrane protein with a molecular weight of 13,000 containing three predicted transmembrane α-helical segments and two amphipathic helices lying along the inner side of the plasma membrane (27). It acts as a homotrimer having three active sites, each consisting of residues belonging to two different subunits (16, 34). The enzyme catalyzes a direct transfer of phosphate from MgATP to diacylglycerol (2), resulting in the formation of phosphatidic acid (PA). The kinase exhibits broad specificity in vitro for lipid substrates, being able to phosphorylate a variety of diacylglycerols and their analogs, monoacylglycerols, as well as ceramide (5, 23, 24, 36).
In bacteria, PA, a major precursor in phospholipid metabolism, is normally synthesized by the sequential acylation of sn-glycero-3-phosphate. PA generation through direct phosphorylation of diacylglycerol by DGK constitutes a minor pathway, which appears to function in the regulation of the diacylglycerol content in the plasma membrane. In E. coli membranes, the diacylglycerol levels are low (approximately 0.5%), yet substantial amounts of neutral lipid are generated during production of membrane-derived oligosaccharide, a periplasmic component involved in osmoregulation. The importance of diacylglycerol recycling via its phosphorylation to PA has been demonstrated in a mutant lacking DGK activity, which was characterized by a marked accumulation of diacylglycerol in the membranes, especially under low osmolarity, a condition stimulating production of membrane-derived oligosaccharide (19, 20). In gram-positive bacteria, diacylglycerol is formed as the result of the transfer of sn-glycero-1-phosphate from phosphatidylglycerol to the growing chain of lipoteichoic acid, a cell wall component anchored in the membrane via a glycolipid (10, 11, 30). The diacylglycerol contents of gram-positive bacteria are generally much higher than in E. coli. In many species, diacylglycerol is further converted to monoglucosyl- and diglucosyldiacylglycerol, glycolipids playing roles in the regulation of the nonbilayer potential of the plasma membrane (9, 33). As in E. coli, diacylglycerol can also be recycled to the main pathway of phospholipid biosynthesis through the action of DGK.
A previous study in our laboratory (37) implicated a gene encoding a putative DGK in the stress responses of the highly cariogenic and acid-tolerant oral bacterium Streptococcus mutans. Disruption of the gene near its 3′ end by insertion of transposon Tn916 resulted in defective growth of the resulting mutant Tn-1 at low pH, elevated temperatures, and high osmolarity. Moreover, another report (8) has described a similar mutation in the dgk gene of another S. mutans strain leading to a defect in production of the lantibiotic mutacin II.
In this report, we present evidence that the product of the S. mutans dgk gene, although belonging to the family of bacterial DGKs, predominantly exhibits specificity toward a lipid substrate other than diacylglycerol, most likely undecaprenol.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. mutans strains GS-5, Tn-1, and TnSp-1 were grown in Todd Hewitt broth or on tryptic soy broth agar plates. E. coli strain DH5α was used for construction of plasmids. E. coli strain RZ6 is a dgk-6 mutant of parental K12 strain R4440 (F− his-4 thr-1 leu-6 stra-136) (19).
Construction of plasmids.
Construction of plasmid pDGK749 was performed as follows. DNA fragments containing the promoter region including the ribosomal binding site of scrB (22) and the coding region of dgk were generated by PCR using the following primers: 5′-CGCTGCAGACGTTATTCATTTTATC-3′ and 5′-GCTCTAGACTCCTAATAATAGTTTATC-3′ for scrB and 5′-GCTCTAGATTAGATGCCTATGGAC-3′ and 5′-GGGTCGACATGATGTCTCCTCTATC-3′ for dgk (their 3′ and 5′ ends, respectively, contained XbaI sites derived from the primers [underlined]). The two fragments were subcloned into plasmid pTrc99A (1) and attached to each other at their respective XbaI sites. The DNA fragment containing both the scrB promoter and the coding sequence of dgk was subsequently excised by using HindIII and NcoI and subcloned into shuttle vector pTS749 (25) and digested with the same restriction enzymes.
The construction of plasmid pDGKSp-1, used for insertional inactivation of dgk, was carried out as follows. An XbaI-SalI DNA fragment containing the dgk gene was excised from plasmid pDGK749 and inserted into a derivative of pUC19, where the EcoRI site had been replaced by HindIII. In the resultant plasmid, a fragment containing the 5′ portion of the dgk gene (between restriction sites XbaI and EcoRI) was replaced with an XbaI-EcoRI fragment from plasmid pHDI (37) containing the same 5′ part of the gene and 274 bp upstream of its start codon, generating plasmid pDGKEX-14. Subsequently, the 3′ portion of the dgk gene (between restriction sites EcoRI and SalI) on pDGKEX-14 was replaced by a PCR-generated DNA fragment containing the 3′ portion of the mutated gene followed by 640 bp of the right arm of the transposon Tn916. The resultant plasmid, pDGK20-3, contained an approximately 1.2-kb region of the Tn-1 chromosome flanking the mutated dgk gene. Finally, the internal portion of the gene, between restriction sites EcoRI and MscI, was replaced with an EcoRI-EcoRV fragment containing the spectinomycin adenyltransferase AAD(9) gene from Enterococcus faecalis (17) positioned in an opposite orientation relative to the dgk gene.
The construction of plasmids for expression of S. mutans dgk in E. coli was carried out as follows. A DNA fragment containing the S. mutans dgk gene was amplified by PCR using a 5′ primer (5′-CCTATGGACTTAAGAGATAATAAG-3′) starting from the second codon and a 3′ primer (5′-GGGTCGACATGATGTCTCCTCTATC-3′; containing a SalI restriction site [underlined]) starting approximately 30 bp downstream from the stop codon. The generated fragment was digested with SalI and inserted into vector pTrc99A (1), which was then digested with NcoI, with protruding ends filled in with Klenow fragment of polymerase I, and finally cut with SalI. The resulting plasmid, pTrc99ADGK-1, contains the wild-type dgk gene under control of the trc promoter. Plasmid pTrc99ADGKTn-31, containing the mutated dgk gene, was constructed by using a similar method except that the 3′ primer (5′-CCGGATCCATGCGGATAACTAGATT-3′) began approximately 30 bp from the end of the right arm of transposon Tn916 and the restriction enzyme BamHI (underlined) was used instead of SalI. Chromosomal DNA of the mutant Tn-1 was used as the template for PCR.
DGK activity assays.
For comparisons of DGK activities in S. mutans and E. coli strains, we measured the incorporation of radioactive phosphorus from [γ-32P]ATP (0.5 Ci/mol) into 1,2-dioleoyl-sn-glycerol or lipids extracted from S. mutans as described by Walsh and Bell (35), except that the reactions carried out in 100 μl were stopped by addition of 60 μl of 2.3% perchloric acid and extraction was downsized accordingly. The membrane preparations containing 10 μg of proteins per sample were used as the source of DGK activities. After reaction, chloroform fractions containing extracted lipids were dried under vacuum and their radioactivities were measured in a scintillation counter (LS5801; Beckman). All samples were assayed in duplicate.
In other experiments, the Biotrak 1,2-diacylglycerol assay system (Amersham) was employed according to the manufacturer's instructions. The E. coli membrane preparation enriched in DGK activity was provided with the kit and used as described in the assay manual. The S. mutans DGK, containing the His6 tag and purified on a Ni-chelate column, was kindly provided by Charles R. Sanders (Case Western Reserve University, Cleveland, Ohio). Approximately 1 μg of the enzyme was used for each reaction. All substrates (diacylglycerols and undecaprenol) were purchased from Sigma.
Isolation of bacterial membranes used as the sources of DGK activity.
Membranes of S. mutans and E. coli were isolated according to the methods of Bender et al. (3) and Walsh et al. (36), respectively.
Extraction of lipids.
For isolation of lipids from S. mutans, the collected cells were resuspended in a solution of 1 M NaCl containing 5% butanol and were extracted with chloroform-methanol by using the method of Bligh and Dyer (4).
Mild alkaline lysis.
Saponification of phospholipids was performed by use of a method modified from that of Steiner et al. (28). Following evaporation under vacuum, phosphorylated lipids were solubilized in 20 μl of toluene-methanol (1:1, vol/vol). Twenty microliters of 0.2 N KOH in methanol was then added, and samples were incubated at 37°C for 20 min. After addition of 20 μl of 1% perchloric acid, lipids were extracted twice with 20 μl of chloroform and separated by thin-layer chromatography (TLC).
TLC.
TLC was performed by using 20- by 20-cm silica gel 60 plates (Merck). The solvent system consisted of chloroform, methanol, and water (25:10:1). Radiolabeled lipids were detected by autoradiography.
RESULTS
Complementation of the mutation in Tn-1 by expression of the intact dgk on a plasmid.
In the mutant Tn-1 strain, the transposon Tn916 was inserted near the 3′ end of the coding sequence of the putative dgk gene, resulting in the replacement of only 10 C-terminal amino acids of the encoded protein with 5 others generated randomly from the sequences of the right arm of the transposon (37). Moreover, the gene is a part of an operon, so insertion of the transposon might have affected expression of other genes due to a polar effect. To confirm that the lack of the product of dgk was specifically responsible for the changed phenotype, the intact gene was introduced in trans on plasmid pDGK749. The growth rates of the wild-type strain GS-5 and the mutant Tn-1, both carrying vector pTS749, and Tn-1 transformed with plasmid pDGK749 were compared. While the growth of mutant Tn-1 ceased at an optical density at 550 nm (OD550) of 0.6 following acidification of culture medium to pH 5.5, introduction of the dgk gene on the plasmid restored growth, under the same conditions, that was indistinguishable from that of the wild-type strain GS-5, which reached an OD550 of 1.3. Similar results were obtained when the growth rates were compared at a temperature of 44°C (data not shown). Thus, it appeared that the lack of the functional gene encoding the putative DGK was solely responsible for the phenotype of the mutant Tn-1.
The mutation in strain Tn-1 results in a significant decrease in lipid kinase activity.
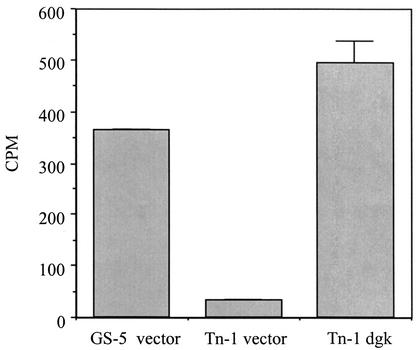
It was of interest to determine whether the phenotypic effects of the mutation were associated with the lack of or reduction of the enzymatic activity encoded by dgk. DGK from E. coli is an integral membrane protein (27). Therefore, we attempted to detect kinase activity in membrane fractions isolated from S. mutans strains used for the complementation experiments described above. We employed a mixed micellar assay protocol (35) utilizing [γ-32P]ATP and 1,2-dioleoyl-sn-glycerol as substrates as well as cardiolipin as a lipid cofactor. Initial experiments failed to generate any chloroform-soluble radioactive products. In contrast, when membranes isolated from E. coli wild-type strain DH5α were used, a significant amount of radioactivity was found to be partitioning into the chloroform phase (data not shown). When the reaction mixture was supplemented with a lipid extract of S. mutans cells, membranes of the wild-type GS-5 strain and mutant Tn-1 carrying dgk on a plasmid exhibited an activity leading to incorporation of 32P into chloroform-soluble material that was higher than that of the mutant with vector only (Fig. 1). To determine whether different substrates or lipid cofactors were needed for supplementation, phosphorylation reactions were carried out by using membranes of strain GS-5 as a source of kinase activity with different amounts of added lipid extracts as well as with omission of either diolein or cardiolipin. The level of radioactivity incorporated into the chloroform phase was proportional to the amount of lipids extracted from S. mutans cells, and the absence of 1,2-dioleoyl-sn-glycerol did not affect the reaction. However, omission of cardiolipin resulted in a decrease in the amount of the chloroform-soluble product to the level observed when no S. mutans lipids were added to the reaction mixture (data not shown).
FIG. 1.
Membrane preparations of S. mutans strains GS-5 and Tn-1 carrying vector pTS749 (GS-5 vector and Tn-1 vector) and Tn-1 carrying plasmid pDGK749 (Tn-1 dgk) were assayed for DGK activity in the presence of lipid extracts from S. mutans. Error bars represent standard errors of the means for reactions performed in duplicate.
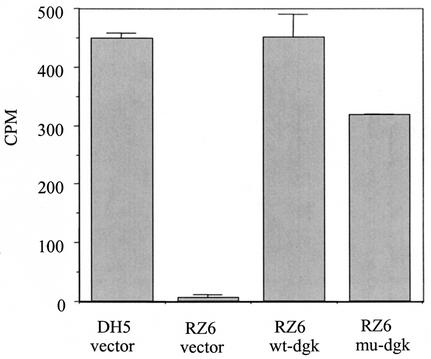
The lipid kinase activity encoded by dgk was also confirmed in membranes of the E. coli dgk mutant RZ6 (19), expressing S. mutans dgk genes (both wild type and mutated) on a plasmid. As described earlier, only membranes of the wild-type DH5α strain were able to support phosphorylation of diolein (data not shown). When lipids extracted from S. mutans were used as the source of substrate, the membranes of both strains DH5α and RZ6, carrying S. mutans dgk, but not that of RZ6 with vector only, exhibited kinase activity (Fig. 2). Interestingly, membranes isolated from RZ6 cells containing the mutated dgk gene from Tn-1 also generated radioactive, chloroform-soluble product, though at somewhat lower levels. The latter result may suggest that insertion of the transposon affected the expression of the gene and consequently that the lack of enzymatic activity in mutant Tn-1 resulted from the absence of encoded protein rather than from changes of its C-terminal amino acids. Alternatively, the observation of enzymatic activity in the E. coli cells may simply be a result of overexpression of the mutated gene whose product normally exhibits very low activity.
FIG. 2.
DGK activities in membrane preparations of E. coli strain DH5α and DGK-deficient mutant RZ6 carrying vector pTrc99A and RZ6 expressing wild-type (wt) and mutated (mu) forms of dgk from S. mutans on plasmids pTrcDGK-1 and pTrcDGKTn-31, respectively. The reactions were performed in the presence of lipid extracts from S. mutans. Error bars represent standard errors of the means for reactions performed in duplicate.
Complete inactivation of the dgk gene.
As stated earlier, the insertion of transposon Tn916 affected only the 3′ terminal region of the dgk gene. Moreover, as indicated above, the mutated gene still expressed lipid kinase activity when present on a plasmid in E. coli cells. This suggested that, although undetected, a residual kinase activity might be present in the Tn-1 cells. To demonstrate whether complete inactivation of the gene affects the viability of the cells or further changes their phenotype, a DNA fragment containing a spectinomycin resistance gene replacing an internal portion of the mutated dgk gene, encompassing approximately 200 bp, was introduced by allelic exchange into the Tn-1 strain. The resultant spectinomycin-resistant, dgk-negative mutant strain was designated TnSp-1. Comparison of the two mutant strains (Tn-1 and TnSp-1) did not reveal significant differences in their behaviors under neutral or acidic pH. This result confirmed that the activity encoded by the dgk gene predominantly affects growth of the cells under stress conditions.
Substrate specificity of the dgk gene product in S. mutans.
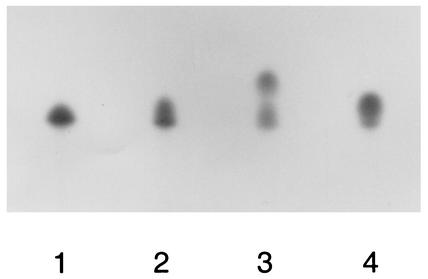
The failure to phosphorylate pure diacylglycerol by membrane preparations of S. mutans suggested the possibility that the lipid kinase encoded by the dgk gene of this microorganism exhibits specificity toward a different lipid substrate than the enzyme from E. coli. To test this hypothesis, we compared the migrations on a TLC plate of phospholipids generated by purified DGK from E. coli (Amersham) and by the membranes isolated from the wild-type S. mutans strain GS-5 (Fig. 3). The product of the reaction catalyzed by the streptococcal kinase migrated faster than the different molecular species of PA generated by the E. coli enzyme from S. mutans lipids or from pure diacylglycerols (Fig. 3). One candidate for a phospholipid exhibiting an apparently slightly lower polarity than PA could be undecaprenyl phosphate.
FIG. 3.
Comparison of TLC separations of phospholipids generated by two different kinases. Lanes 1, 2, and 4, TLC separations of phospholipids generated by E. coli kinase from diacylglycerol (16:0), from S. mutans lipids, and from diacylglycerol (18:1), respectively; lane 3, separation of products of reactions catalyzed by membranes isolated from S. mutans and by purified DGK from E. coli by using S. mutans lipids as substrates. The products of both reactions were mixed prior to TLC separation.
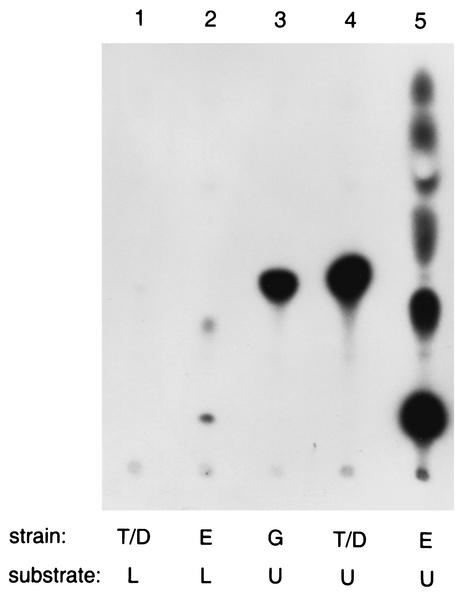
We then attempted to phosphorylate preparations of undecaprenol by the membranes isolated from the wild-type GS-5 strain, from mutant Tn-1, and from the mutant carrying the S. mutans dgk gene on a plasmid. The DGK from E. coli, known to be unable to phosphorylate undecaprenol (5), was used as a control. TLC separation of radioactive chloroform-soluble products along with the products of phosphorylation of lipids extracted from S. mutans cells is shown in Fig. 4. S. mutans DGK generated from undecaprenol large amounts of a phospholipid comigrating with a product formed by the same enzyme from isolated lipids (Fig. 4, lanes 3, 4, and 1, respectively). In contrast, membranes of the mutant Tn-1 were unable to form any significant amount of radioactive phospholipid from undecaprenol or extracted lipids (data not shown). As expected, E. coli DGK stimulated formation of PA from the lipid extract (lane 2), but surprisingly, it generated PA and other unidentified phospholipids from preparations of undecaprenol as well (lane 5), although none of them comigrated with the putative undecaprenyl phosphate formed by the streptococcal kinase. Apparently, the commercial preparation of undecaprenol, which was used as a substrate, contained substantial amounts of diacylglycerol and possibly other lipids, and the question of whether undecaprenol or an unusual form of diacylglycerol are phosphorylated by S. mutans kinase was therefore not resolved.
FIG. 4.
Phosphorylation of the lipid extract from S. mutans (L) or of undecaprenol (U). The sources of lipid kinase activities used were isolated membranes of S. mutans strains: GS-5 (G) or Tn-1 expressing S. mutans dgk on a plasmid (T/D) or purified DGK from E. coli (E).
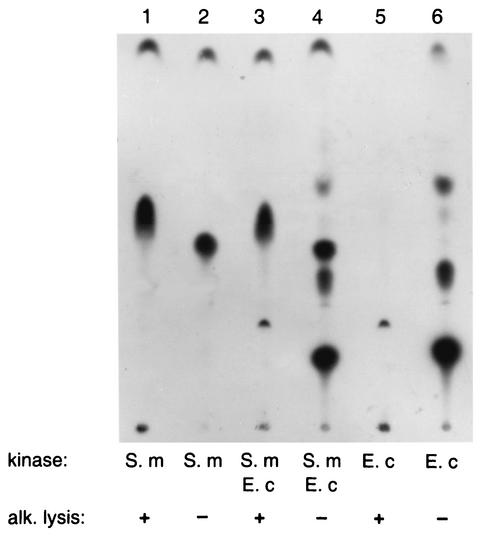
In contrast to glycerophospholipids, polyprenols, including undecaprenol and undecaprenyl phosphate, are resistant to saponification, a reaction separating hydrophobic fatty-acid chains from water-soluble glycerophosphate. To determine whether the products of phosphorylation by the two kinases differ in their susceptibilities to saponification, the preparation of undecaprenol was phosphorylated by using E. coli DGK and the membranes of S. mutans. Radioactive products of the reactions were then deacylated by mild alkaline lysis (28), extracted with chloroform-methanol, and separated by TLC along with untreated samples. As shown in Fig. 5, none of the products generated by the E. coli DGK can be seen on autoradiograms following saponification, as expected for glycerophospholipids, whereas putative undecaprenyl phosphate remains intact after the same treatment. The slightly different migration of the alkali-treated product (Fig. 5, lane 1) compared with that of the nontreated sample (lane 2) can be explained by the fact that the former was not neutralized after alkaline lysis, a procedure which may change some physicochemical properties of a phospholipid and, as a consequence, its behavior during chromatographic separation. In addition, the products of both reactions were mixed together and separated by TLC directly (lane 4) or after alkaline lysis (lane 3), yielding the same results as for the individual products.
FIG. 5.
Saponification of phospholipids generated from undecaprenol by membranes isolated from S. mutans (S. m) or by purified DGK from E. coli (E. c).
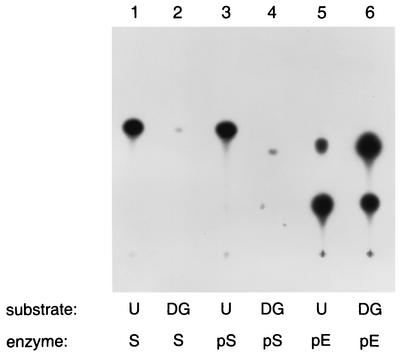
Finally, more direct evidence that the enzyme encoded by the dgk gene from S. mutans is responsible for the altered lipid kinase activity observed in the membranes isolated from this microorganism was obtained after the protein, purified to near homogeneity from an E. coli clone expressing His tag-tagged dgk from S. mutans, was used to phosphorylate commercial preparations of undecaprenol and diacylglycerol (16:0). In parallel, purified E. coli DGK and S. mutans membranes were used as in the previous experiments. As shown in Fig. 6, purified streptococcal kinase (lane 3) generated a phospholipid from undecaprenol, appearing on the TLC autoradiogram as a spot migrating identically with that formed by membranes from S. mutans (lane 1) and distinct from PA and other phospholipids generated by E. coli DGK (lanes 5 and 6). The reaction carried out in the presence of diacylglycerol and membranes isolated from S. mutans (lane 2) led to formation of a small amount of putative undecaprenyl phosphate, probably from the endogenous substrate present in the membranes. In contrast, incubation of purified kinase from S. mutans with diacylglycerol resulted in formation of only a trace amount of PA (lane 4).
FIG. 6.
Generation of phospholipids from undecaprenol (U) and diacylglycerol (DG) by S. mutans membranes (S), by purified kinase from S. mutans (pS), and by purified DGK from E. coli (pE).
The effects of mutation on resistance to bacitracin.
Undecaprenyl phosphate plays a key role in the synthesis of peptidoglycan and other bacterial cell wall components as a lipid carrier compartmentalizing nascent polymer chains to the plasma membrane during their assembly. After each cycle, the lipid carrier is released in the form of undecaprenyl pyrophosphate, which is subsequently dephosphorylated to the monophosphate. Dephosphorylation is blocked by the antibiotic bacitracin, leading to depletion of the lipid carrier and cessation of cell growth (26). Although recycling and de novo synthesis, both involving bacitracin-sensitive dephosphorylation, constitute a major source of the lipid carrier supply, another minor pathway, phosphorylation of free undecaprenol, has been postulated based on the discovery of the activity of undecaprenol kinase in some bacteria (14, 18, 21). The levels of this activity appear to correlate with the degree of resistance to bacitracin (6, 7). Therefore, we could expect that if the major physiological role played by the product of the S. mutans dgk gene is that of undecaprenol kinase, then the mutant Tn-1 and TnSp-1 strains should be more sensitive to bacitracin than is the wild-type parental strain GS-5. Indeed, although the initial growth rates of all three strains were very low in the presence of 2 U of bacitracin/ml in liquid broth compared with the growth without antibiotic (data not shown) after 24 h, the cultures of the wild-type strain with and without bacitracin reached comparable OD550 values (1.1 and 1.3, respectively). In contrast, the presence of antibiotic in the medium completely abolished the growth of both mutants (OD550 values below 0.2). Similar effects of bacitracin were observed on agar medium, where strains GS-5, Tn-1, and Tn-1 expressing dgk on a plasmid were compared. Their survivals with respect to the growth without antibiotic were 10, 0.0001, and 15%, respectively.
DISCUSSION
One of the most important properties contributing to the cariogenicity of S. mutans, the major etiological agent of dental caries, is its relatively high tolerance to low pH. To obtain further insight into bacterial physiology under acidic conditions, we analyzed a previously isolated S. mutans mutant Tn-1 strain characterized by sensitivity to low pH and other environmental stresses. Of particular interest was the fact that the transposon-generated mutation affected a gene expressing a protein with amino acid homology with the DGK from E. coli, pointing to a possibly novel mechanism of response of the streptococci to changes in the environment.
We confirmed that the defect in the dgk gene and more specifically the lack of the lipid kinase activity of its product are solely responsible for the stress-sensitive phenotype of the mutant. Further analysis revealed that despite its amino acid sequence similarity to the class of bacterial DGKs, the product of the dgk gene in S. mutans predominantly exhibits an activity distinct from its E. coli counterpart, very likely that of an undecaprenol kinase, although the purified enzyme was still able to phosphorylate diacylglycerol with very low efficiency.
Although the identity of undecaprenol as the primary substrate for streptococcal kinase cannot be established unambiguously, it is supported by several lines of evidence. A preparation of undecaprenol was efficiently phosphorylated by S. mutans membranes as well as by purified DGK from this microorganism, and the product of the reaction was resistant to saponification. The substrate of this kinase was not recognized by DGK from E. coli, an enzyme exhibiting an otherwise broad specificity. Finally, the marked difference in resistance to bacitracin between the wild-type and mutant strains is consistent with the proposed role of undecaprenol kinases in bypassing the block in synthesis of the lipid carrier imposed by this antibiotic.
The alignment of the amino acid sequences revealed 27.5% identity and 63.8% similarity between the products of the dgk genes in S. mutans and E. coli (37), suggesting their common ancestral origin. It would be of interest to determine how the structural differences between the two enzymes relate to their different substrate specificities and whether the ability to phosphorylate undecaprenol is a unique feature of DGK from S. mutans or rather is more common among the bacterial enzymes. It should be noted that another gene, bacA, originally isolated from E. coli through selection for clones exhibiting resistance to bacitracin, has been proposed to encode undecaprenol kinase activity (6, 7). Moreover, a BLAST search of the unfinished genome sequence of S. mutans (University of Oklahoma) revealed the existence of a bacA homologue in this species as well. Therefore, the actual enzymatic activity of the bacA product, especially in S. mutans, and the mechanism by which it confers resistance to bacitracin need to be clarified.
The proposed dependence of growth under stress conditions on the activity of undecaprenol kinase observed in S. mutans points to synthesis of peptidoglycan or other cell wall polymers as part of the response of this bacterium to changes in the environment. Synthesis of many bacterial wall components is strictly limited by the availability of the lipid carrier undecaprenyl phosphate. The rates of de novo synthesis and recycling of lipid carrier are probably sufficient to maintain its steady-state levels, whereas direct phosphorylation of free isoprenoid alcohol, which generally accounts for a large percentage of all polyprenols in bacterial cells (13, 31, 32), may constitute a means for its rapid expansion when needed to increase the production of a specific component of the cell wall. Consequently, we can hypothesize that in S. mutans such a component plays an important role necessary for growth under stress conditions. Although the exact mechanism of the stress response associated with the function of putative undecaprenol kinase is unknown, it appears to be unrelated to known mechanisms of defense against acidification (12, 15, 29). This notion is supported by the fact that, apart from impaired growth under low pH, we were unable to detect any defect in the acid adaptation response of the mutant Tn-1 (our unpublished results).
Acknowledgments
We thank C. R. Sanders for very helpful suggestions and discussions as well as for the gift of purified DGK from S. mutans.
This project was supported in part by National Institutes of Health grants DE03258 and DE10711.
Editor: V. J. DiRita
REFERENCES
- 1.Amann, E., B. Ochs, and K. J. Abel. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301-315. [DOI] [PubMed] [Google Scholar]
- 2.Badola, P., and C. R. Sanders II. 1997. Escherichia coli diacylglycerol kinase is an evolutionarily optimized membrane enzyme and catalyzes direct phosphoryl transfer. J. Biol. Chem. 272:24176-24182. [DOI] [PubMed] [Google Scholar]
- 3.Bender, G. R., S. V. Sutton, and R. E. Marquis. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 5.Bohnenberger, E., and H. Sandermann, Jr. 1979. Diglyceride kinase from Escherichia coli. Purification in organic solvent and some properties of the enzyme. Eur. J. Biochem. 94:401-407. [DOI] [PubMed] [Google Scholar]
- 6.Cain, B. D., P. J. Norton, W. Eubanks, H. S. Nick, and C. M. Allen. 1993. Amplification of the bacA gene confers bacitracin resistance to Escherichia coli. J. Bacteriol. 175:3784-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalker, A. F., K. A. Ingraham, R. D. Lunsford, A. P. Bryant, J. Bryant, N. G. Wallis, J. P. Broskey, S. C. Pearson, and D. J. Holmes. 2000. The bacA gene, which determines bacitracin susceptibility in Streptococcus pneumoniae and Staphylococcus aureus, is also required for virulence. Microbiology 146:1547-1553. [DOI] [PubMed] [Google Scholar]
- 8.Chen, P., J. Novak, F. Qi, and P. W. Caufield. 1998. Diacylglycerol kinase is involved in regulation of expression of the lantibiotic mutacin II of Streptococcus mutans. J. Bacteriol. 180:167-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowhan, W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66:199-232. [DOI] [PubMed] [Google Scholar]
- 10.Emdur, L., and T. Chiu. 1975. The role of phosphatidylglycerol in the in vitro biosynthesis of teichoic acid and lipoteichoic acid. FEBS Lett. 55:216-219. [DOI] [PubMed] [Google Scholar]
- 11.Glaser, L., and B. Lindsay. 1974. The synthesis of lipoteichoic acid carrier. Biochem. Biophys. Res. Commun. 59:1131-1136. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton, I. R., and G. Svensater. 1998. Acid-regulated proteins induced by Streptococcus mutans and other oral bacteria during acid shock. Oral Microbiol. Immunol. 13:292-300. [DOI] [PubMed] [Google Scholar]
- 13.Higashi, Y., J. L. Strominger, and C. C. Sweeley. 1970. Biosynthesis of the peptidoglycan of bacterial cell walls. XXI. Isolation of free C55-isoprenoid alcohol and of lipid intermediates in peptidoglycan synthesis from Staphylococcus aureus. J. Biol. Chem. 245:3697-3702. [PubMed] [Google Scholar]
- 14.Kalin, J. R., and C. M. Allen, Jr. 1979. Characterization of undecaprenol kinase from Lactobacillus plantarum. Biochim. Biophys. Acta 574:112-122. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi, H., N. Murakami, and T. Unemoto. 1982. Regulation of the cytoplasmic pH in Streptococcus faecalis. J. Biol. Chem. 257:13246-13252. [PubMed] [Google Scholar]
- 16.Lau, F. W., X. Chen, and J. U. Bowie. 1999. Active sites of diacylglycerol kinase from Escherichia coli are shared between subunits. Biochemistry 38:5521-5527. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc, D. J., L. N. Lee, and J. M. Inamine. 1991. Cloning and nucleotide base sequence analysis of a spectinomycin adenyltransferase AAD(9) determinant from Enterococcus faecalis. Antimicrob. Agents Chemother. 35:1804-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poxton, I. R., J. A. Lomax, and I. W. Sutherland. 1974. Isoprenoid alcohol kinase—a third butanol-soluble enzyme in Klebsiella aerogenes membranes. J. Gen. Microbiol. 84:231-233. [DOI] [PubMed] [Google Scholar]
- 19.Raetz, C. R., and K. F. Newman. 1978. Neutral lipid accumulation in the membranes of Escherichia coli mutants lacking diglyceride kinase. J. Biol. Chem. 253:3882-3887. [PubMed] [Google Scholar]
- 20.Raetz, C. R., and K. F. Newman. 1979. Diglyceride kinase mutants of Escherichia coli: inner membrane association of 1,2-diglyceride and its relation to synthesis of membrane-derived oligosaccharides. J. Bacteriol. 137:860-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandermann, H., Jr., and J. L. Strominger. 1972. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J. Biol. Chem. 247:5123-5131. [PubMed] [Google Scholar]
- 22.Sato, Y., and H. K. Kuramitsu. 1988. Sequence analysis of the Streptococcus mutans scrB gene. Infect. Immun. 56:1956-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider, E. G., and E. P. Kennedy. 1973. Phosphorylation of ceramide by diglyceride kinase preparations from Escherichia coli. J. Biol. Chem. 248:3739-3741. [PubMed] [Google Scholar]
- 24.Schneider, E. G., and E. P. Kennedy. 1976. Partial purification and properties of diglyceride kinase from Escherichia coli. Biochim. Biophys. Acta 441:201-212. [DOI] [PubMed] [Google Scholar]
- 25.Shiroza, T., and H. K. Kuramitsu. 1993. Construction of a model secretion system for oral streptococci. Infect. Immun. 61:3745-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siewert, G., and J. L. Strominger. 1967. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in biosynthesis of the peptidoglycan of bacterial cell walls. Proc. Natl. Acad. Sci. USA 57:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, R. L., J. F. O'Toole, M. E. Maguire, and C. R. Sanders II. 1994. Membrane topology of Escherichia coli diacylglycerol kinase. J. Bacteriol. 176:5459-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner, S., S. F. Conti, and R. L. Lester. 1969. Separation and identification of the polar lipids of Chromatium strain D. J. Bacteriol. 98:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svensater, G., U. B. Larsson, E. C. Greif, D. G. Cvitkovitch, and I. R. Hamilton. 1997. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 12:266-273. [DOI] [PubMed] [Google Scholar]
- 30.Taron, D. J., W. C. Childs III, and F. C. Neuhaus. 1983. Biosynthesis of d-alanyl-lipoteichoic acid: role of diglyceride kinase in the synthesis of phosphatidylglycerol for chain elongation. J. Bacteriol. 154:1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorne, K. J. I. 1973. Identification of prenol intermediates of wall biosynthesis in growing cells of Lactobacillus plantarum. J. Bacteriol. 116:235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umbreit, J. N., K. J. Stone, and J. L. Strominger. 1972. Isolation of polyisoprenyl alcohols from Streptococcus faecalis. J. Bacteriol. 112:1302-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vikstrom, S., L. Li, and A. Wieslander. 2000. The nonbilayer/bilayer lipid balance in membranes. Regulatory enzyme in Acholeplasma laidlawii is stimulated by metabolic phosphates, activator phospholipids, and double-stranded DNA. J. Biol. Chem. 275:9296-9302. [DOI] [PubMed] [Google Scholar]
- 34.Vinogradova, O., P. Badola, L. Czerski, F. D. Sonnichsen, and C. R. Sanders II. 1997. Escherichia coli diacylglycerol kinase: a case study in the application of solution NMR methods to an integral membrane protein. Biophys. J. 72:2688-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh, J. P., and R. M. Bell. 1986. sn-1,2-Diacylglycerol kinase of Escherichia coli. Mixed micellar analysis of the phospholipid cofactor requirement and divalent cation dependence. J. Biol. Chem. 261:6239-6247. [PubMed] [Google Scholar]
- 36.Walsh, J. P., L. Fahrner, and R. M. Bell. 1990. sn-1,2-diacylglycerol kinase of Escherichia coli. Diacylglycerol analogues define specificity and mechanism. J. Biol. Chem. 265:4374-4381. [PubMed] [Google Scholar]
- 37.Yamashita, Y., T. Takehara, and H. K. Kuramitsu. 1993. Molecular characterization of a Streptococcus mutans mutant altered in environmental stress responses. J. Bacteriol. 175:6220-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]