Abstract
Plasmodium berghei-infected mice, a well-recognized model of experimental cerebral malaria (ECM), exhibit many of the hallmarks of a systemic inflammatory response, with organ damage in brain, lung, and kidneys. Identification of the molecules mediating pathogenesis of the inflammatory response, such as leukocyte adhesion, may lead to new therapies. Indeed, mice lacking the cell adhesion molecule P-selectin were significantly (P = 0.005) protected from death due to P. berghei malaria compared with C57BL/6 controls despite similar parasitemia (P = 0.6) being found in both groups of mice. P-selectin levels assessed by the quantitative dual radiolabeled monoclonal antibody technique increased significantly (P < 0.05) in several organs in C57BL/6 mice infected with P. berghei, supporting the concept of a systemic inflammatory response mediating malarial pathogenesis. Intravital microscopic analysis of the brain microvasculature demonstrated significant (P < 0.001) leukocyte rolling and adhesion in brain venules of P. berghei-infected mice compared with those found in uninfected controls. The maximum leukocyte adhesion occurred on day 6 of P. berghei infection, when the mice become moribund and exhibit marked vascular leakage into the brain, lung, and heart. However, P-selectin levels were significantly (P < 0.005) increased in brain, lung, and kidneys during P. berghei malaria in ECM-resistant BALB/c mice compared with those found in uninfected BALB/c controls, indicating that increased P-selectin alone is not sufficient to mediate malarial pathogenesis. Leukocyte adhesion to brain microvessels of P-selectin-deficient mice with P. berghei malaria was similar to that observed in control mice. Collectively, these results indicate that P-selectin is important for the development of malarial pathogenesis but is not required for leukocyte adhesion in brain.
Insults to the body, such as localized infection, surgery, or ischemia and/or reperfusion, lead to a limited inflammatory response as part of the healing process. However, major or prolonged insults and autoimmune diseases are associated with an uncontrolled systemic inflammatory response in which leukocytes damage certain tissues. Indeed, inflammation is evident in rheumatoid arthritis, systemic lupus erythematosus, vasculitis, and inflammatory bowel disease and has led to targeted anti-inflammatory interventions to ameliorate these diseases. A critical step targeted for intervention in systemic inflammatory response diseases is the adhesion of leukocytes to the endothelial barrier and their migration into the affected tissue. Thus, defining the molecular mechanisms whereby leukocytes roll on, adhere to, and extravasate through the vascular endothelium is important for the development of therapy in a number of important human diseases.
In order to adhere to the vascular endothelium, leukocytes must overcome the potent hydrodynamic forces of the circulatory system. To overcome these forces, leukocyte adhesion is a complex multistep process (reviewed in reference 40). Infection or an inflammatory stimulus causes a rapid increase in P-selectin on the surface of the endothelial cell from preformed storage vesicles (Weibel-Palade bodies) and increased transcription of P-selectin mRNA (41). P-selectin binds to sialylated Lewis X antigen, which is contained within P-selectin glycoprotein ligand 1 expressed on the surface of leukocytes and platelets (10). This binding causes the rolling of leukocytes on the vascular endothelium and allows these cells to interact with chemokines bound to endothelial cell glycosaminoglycans (3). Chemokines binding to their receptors on the leukocytes cause “inside-out” signaling and the activation of integrin molecules on the surface of the leukocyte to assume a conformation with a higher affinity for its ligand on endothelial cells (40). This high-affinity interaction arrests the leukocyte on the endothelial cell. Thus, the first step in the complex adhesion process is the binding of leukocytes to P-selectin on endothelial cells.
Because (i) analysis of tissue sections of the brain and lung during experimental cerebral malaria (ECM) indicates that leukocytes adhere to microvasculature (7, 35) and (ii) leukocytes are required for the development of ECM (15, 36, 42), we investigated whether P-selectin is required for leukocyte adherence in brain vasculature as it is in other vascular beds under different inflammatory stimuli. There are two animal models of ECM: the Plasmodium berghei- and Plasmodium yoelii 17XL-infected mouse (9, 29, 37). The species of Plasmodium that infect mice are phylogenetically distinct from Plasmodium falciparum, the most virulent species that infects humans. However, P. berghei infection results in acute respiratory distress with lactic acidosis and nephritis, as well as impaired consciousness (4, 9, 38). Both P. yoelii- and P. berghei-infected erythrocytes adhere to the brain microvasculature in mice (14, 19).
The relevance of results from murine model of malaria is often questioned because of our poor understanding of human malarial pathogenesis (reviewed in references 5, 6, and 31). The predominant hypothesis is the mechanical hypothesis in which parasitized erythrocytes bind to the endothelium of cerebral blood vessels via endothelial P-selectin, ICAM-1, VCAM-1, and PECAM-1. The resulting obstruction of blood flow leads to decreased distribution of nutrients and O2 to the brain and impaired removal of toxic waste products like lactic acid. The inflammatory hypothesis postulates that proinflammatory cytokines (tumor necrosis factor alpha and gamma interferon) increase cell adhesion molecule levels in the vasculature, leading to leukocyte adherence and the release of toxic factors (31). Both the mechanical and inflammatory hypotheses may explain acute respiratory distress and nephritis observed in patients with severe P. falciparum malaria. These are not, however, mutually exclusive hypotheses because the increased levels of vascular cell adhesion molecules induced by proinflammatory cytokines also increase parasite sequestration via these molecules (5, 6, 31). Shock in humans with P. falciparum malaria and by implication a systemic inflammatory response is, according to the World Health Organization, a poor prognostic indicator (22).
In these studies, we elected to focus on the role of the inflammatory component of malaria and to compare cerebral inflammation with our understanding of inflammation in other vascular beds. We selected P. berghei over P. yoelii because (i) there is marked systemic inflammation with P. berghei, whereas inflammation is less with P. yoelii, and (ii) mice infected with P. berghei exhibit impaired consciousness, whereas P. yoelii-infected mice do not (9, 29, 37). We performed intravital microscopy analysis of leukocyte adhesion in the brain microvasculature of P. berghei-infected mice because this technique assesses leukocyte rolling and adhesion directly in the circulation of a live animal and under flow. Because P-selectin is an important leukocyte cell adhesion molecule, we also determined whether P-selectin is required for experimental malaria pathogenesis and then assessed the amount of endothelial P-selectin by the dual radiolabeled monoclonal antibody (MAb) technique in the brains and selected other organs in ECM-susceptible C57BL/6 mice and in ECM-resistant BALB/c mice (25, 29). Comparison of vascular P-selectin levels in these two strains of mice with P. berghei malaria allows us to determine whether differences in P-selectin may account for survival rates that differ from those found in mice with experimental malaria. Analysis of cell adhesion molecule levels by others was performed with semiquantitative immunohistochemistry (25, 28, 34), whereas the dual radiolabeled MAb technique directly assesses protein on the vasculature. Finally, we determined whether P-selectin is required during malaria for leukocyte adhesion, which is required for ECM pathogenesis.
We report that P-selectin-deficient mice were significantly (P = 0.005) protected from P. berghei pathogenesis, compared with C57BL/6 controls, despite both groups of mice having similar (P = 0.6) parasitemia on day 6 of infection. In addition, we observed a marked increase in P-selectin levels in the vascular beds of several organs (including the brain, lung, and kidneys) and marked leukocyte adhesion in brain microvessels during P. berghei malaria. In contrast to our expectations, we observed (i) similar increases in endothelial P-selectin in ECM-susceptible and -resistant strains of mice and (ii) marked leukocyte adhesion in mice lacking P-selectin expression. We therefore conclude that P-selectin contributes to severe experimental malaria but is not required for leukocyte rolling and adhesion in the brain microvasculature.
MATERIALS AND METHODS
Parasite, infection, and treatment of mice.
Mice were infected with lactate dehydrogenase virus-free P. berghei ANKA by intraperitoneal injection of 106 parasitized erythrocytes obtained from a source mouse. The parasite was maintained as a frozen stabilate as described previously (18). The parasitemia in P. berghei-infected mice was assessed by counting in Giemsa-stained thin blood films the number of parasitized erythrocytes in 200 to 1,000 erythrocytes. Female C57BL/6, P-selectin-deficient (P-sel−/−) mice on a C57BL/6 background and BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, Me.) at 4 to 5 weeks of age and were provided autoclaved food and sterile water ad libitum in Association for Assessment of Laboratory Animal Care-approved, specific-pathogen-free housing at Louisiana State University Health Sciences Center. In each experiment, age- and sex-matched groups of between three and eight mice were used and the animals were between 6 and 10 weeks of age. All procedures were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center.
Analysis of P-selectin on endothelium.
Histology and immunostaining of P-selectin were performed as described previously (20). Briefly, mice were injected intravenously with either 0.1 mg of anti-P-selectin MAb (clone RB40.34; Pharmingen, San Diego, Calif.) or 0.1 mg of rat immunoglobulin G (Accurate Scientific) and were allowed to circulate for 10 min. The brain was removed, placed in Zamboni's fixative, snap-frozen in liquid nitrogen, and then cut into 10-μm-thick sections. The brain sections were incubated with 10% donkey serum to reduce nonspecific binding, washed with phosphate-buffered saline, and then incubated with donkey anti-rat secondary antibody conjugated to Cy3 fluorochrome for 1 h in a humid chamber. The tissue sections were washed and mounted in Mowiol-glycerol containing 2.5% 1,4-diazabicyclo[2.2.2]octane (pH 8.5). Images of anti-P-selectin-labeled brain microvasculature were acquired with a SenSys digital camera on a Nikon Eclipse TE 300 fluorescence microscope and were processed by using the Metamorph program. All samples were processed simultaneously, and the settings for the digital camera were kept constant to minimize differences due to sample processing and acquisition.
Analysis of P-selectin levels in selected organs by the dual radiolabeled MAb technique was performed as described earlier (32). Briefly, C57BL/6 mice were anesthetized by a subcutaneous injection of 150 mg of ketamine/kg of body weight and 7.5 mg of xylazine/kg. The right jugular vein and right carotid artery were cannulated with polyethylene tubing (PE-10), and 500,000 ± 100,000 cpm (0.5 to 5 mg in 200 μl) of specific 125I-anti-P-selectin MAb (clone RB40.34) and nonbinding 131I-anti-keyhole limpet hemocyanin MAb (clone A110-1; Pharmingen) was injected via the jugular vein catheter. Two hundred microliters of 0.9% saline containing 50 U of heparin was injected and allowed to circulate for 5 min. A blood sample (200 μl) was then obtained through the carotid artery to determine 125I and 131I levels in serum. The circulatory system of the animal was washed to remove nonadherent antibody by perfusing with bicarbonate-buffered saline (6 ml) through the jugular vein catheter, and then the thoracic inferior vena cava was cut and flushed with 15 ml of bicarbonate-buffered saline through the carotid artery catheter. Selected organs were dissected from the animal and weighed, and the levels of radioactivity were immediately assessed by a scintillation counter (Wizard 3; Wallac, Turku, Finland). P-selectin levels are expressed as nanograms of MAb/gram of tissue and are determined by
 |
Assessment of rolling and adhesion in pial microvessels.
The mice were anesthetized by intraperitoneal injection of ketamine (200 mg/kg) plus xylazine (10 mg/kg). The jugular vein was cannulated with polyethylene tubing in order to administer rhodamine 6G and sodium pentobarbital for euthanasia. An incision was made over the skull, which was removed by creating a circular opening with a handheld surgical drill. The exposed brain was suffused and moistened with phosphate-buffered saline at 35°C. The body temperature was measured and maintained between 36 and 37°C by using a heating pad with a rectal thermometer. The mouse was then placed on a Nikon upright fluorescence microscope with an intensified camera (Hamamatsu Photonics, Toyoda-cho, Japan), and the ×10-magnification images were recorded on videotape for offline analysis. Five nonoverlapping regions were analyzed for each mouse, and each region was videotaped for at least 5 min (approximately 25 min total). At least four mice were analyzed for each group. Leukocytes were fluorescence labeled by injection of 0.025% rhodamine 6G (0.01 ml). Vessel diameters were determined by using an offline analysis system. Arterioles with diameters of between 20 and 40 μm and postcapillary venules between 10 and 17 μm (small) and between 30 and 50 μm (large) were analyzed for rolling and adherence. Rolling leukocytes were cells moving at a velocity significantly lower than the centerline velocity of the microvessel; their numbers are provided as cells per second per vessel diameter. Adherent cells were cells that did not move within a 30-s observation period.
Statistical analysis.
Analysis of variance with the Statview 5.0 program (SAS Institute) was performed to compare all measurements. P values of less than 0.05 were considered significant. The mean and standard deviation of the parasitemia are reported because this is an average of single measurements, whereas mean and standard error of the mean (SEM) are reported for intravital microscopy measurements where the average of the mean number of cells in several vessels is calculated. Differences between Kaplan-Meier survival curves of groups of mice were compared by the log rank test.
RESULTS
On day 2 of P. berghei infection, the mice appear healthy and have few, if any, parasites observable in Giemsa-stained, thin blood films. On day 4, adequately infected mice have parasitemia ranging between 0.5 and 5% but have no overt clinical symptoms of malaria. During our experiments, virtually all infected C57BL/6 mice develop severe malaria between days 5 and 7 of infection, with the majority occurring on day 6. The mice become lethargic, breathe rapidly, lose their righting and gripping reflexes, and are moribund. Survival experiments are terminated on day 12 of P. berghei infection because this is considered by most investigators to be the last time point at which the infected mice will develop cerebral malaria. None of the 10 P. berghei-infected BALB/c mice became moribund prior to day 12 of infection, verifying that they are resistant to P. berghei malaria.
P-selectin functions in pathogenesis of P. berghei malaria.
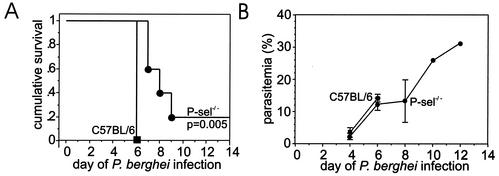
To determine whether P-selectin functions in malarial pathogenesis, we assessed the survival and parasitemia of P-selectin-deficient mice infected with P. berghei. All four C57BL/6 control mice succumbed to malaria on day 6 of P. berghei malaria, whereas all five P-sel−/− mice survived beyond day 6 and one P-sel−/− mouse survived beyond day 12 of infection (Fig. 1). The average parasitemia was similar in the P-selectin-deficient mice and in infected C57BL/6 controls on day 4 (P = 0.3) and day 6 (P = 0.6) of P. berghei infection (Fig. 1B), indicating that the difference in survival was not due to inadequate infection or altered replication of the parasite. Similar results were obtained in a replicate experiment. When both experiments are combined, P-selectin-deficient mice (n = 9) exhibited significantly (P < 0.001) increased survival compared with C57BL/6 controls (n = 9).
FIG. 1.
Effect of P-selectin deficiency (A) on the survival of groups of mice during P. berghei malaria. The average parasitemia and standard deviation of the groups of mice are shown (B).
Markedly increased vascular P-selectin in brain and other organs during P. berghei malaria.
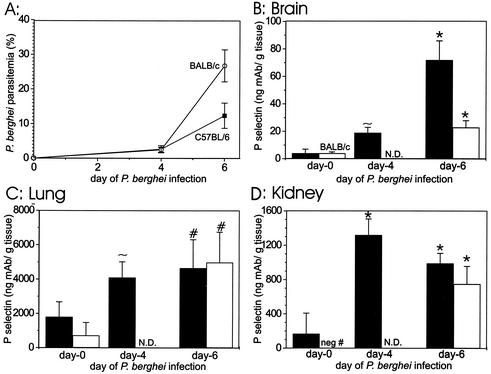
To determine whether P-selectin on the endothelium is increased systemically in several organs or just in the brain during the pathogenesis of experimental malaria, we assessed P-selectin levels on the endothelium of selected organ beds by the dual radiolabeled MAb technique by using groups of five C57BL/6 mice on days 0, 4, and 6 of P. berghei infection. Vascular P-selectin levels are significantly (P < 0.05) increased in the brains, lungs, and kidneys of C57BL/6 mice infected with P. berghei, compared with levels in uninfected controls (16.2-, 2.6-, and 5.9-fold with P = 0.02, 0.02, and <0.0001, respectively) (Fig. 2). The increase in P-selectin was maximal on day 6 of infection, when the animals were moribund. In addition, significant (P < 0.05) increases in vascular P-selectin levels during P. berghei malaria in C57BL/6 mice also occurred in pancreas, thymus, heart, gastrointestinal tract, and muscle (Table 1). The parasitemia was 2.7% ± 1.0% on day 4 and 12.4% ± 3.7% on day 6 of P. berghei infection (Fig. 2A), indicating that the mice were adequately infected. This experiment was repeated with similar results.
FIG. 2.
P. berghei parasitemia in groups of either ECM-susceptible C57BL/6 mice (▪)or in ECM-resistant BALB/c mice (○) (A) and P-selectin in brain (B), lung (C), and kidney (D) during the course of P. berghei malaria in either ECM-susceptible C57BL/6 mice (filled bar) or in ECM-resistant BALB/c mice (open bar). For C57BL/6 mice, n = 8, 4, and 4 on days 0, 4, and 6, respectively; for BALB/c mice, n = 4 for all groups. Shown is the mean plus or minus standard deviation. ∼, P < 0.05; #, P < 0.005; and *, P < 0.0005 for the comparison with uninfected strain-matched controls. N.D., not done.
TABLE 1.
P-selectin in selected organs during the course of P. berghei malaria in groups of ECM-susceptible C57BL/6 mice (n = 8, 4, and 4 on days 0, 4, and 6, respectively) (mean plus or minus standard deviation)
| Tissue | Level of P-selectina on:
|
Change (n-fold) for:
|
P for:
|
||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 4 | Day 6 | Day 4 vs day 0 | Day 6 vs day 0 | Day 4 vs day 0 | Day 6 vs day 0 | |
| Heart | 13 ± 15 | 61 ± 27 | 93 ± 23 | 4.8 | 7.3 | 0.007 | <0.0001 |
| Pancreas | 32 ± 27 | 143 ± 47 | 176 ± 30 | 4.5 | 5.5 | 0.0005 | <0.0001 |
| Mesentery | 19 ± 20 | 111 ± 32 | 141 ± 28 | 5.8 | 7.4 | 0.0002 | <0.0001 |
| Stomach | 73 ± 55 | 277 ± 59 | 512 ± 98 | 3.8 | 7.0 | 0.001 | <0.0001 |
| Small bowel | 20 ± 14 | 94 ± 36 | 139 ± 26 | 4.7 | 6.9 | 0.0008 | <0.0001 |
| Colon | 34 ± 27 | 132 ± 27 | 172 ± 12 | 3.9 | 5.1 | 0.005 | 0.002 |
| Cecum | 27 ± 19 | 115 ± 40 | 129 ± 55 | 4.3 | 4.8 | 0.005 | <0.0001 |
| Muscle | 29 ± 30 | 150 ± 40 | 210 ± 52 | 5.1 | 7.1 | 0.0008 | <0.0001 |
| Thymus | 56 ± 72 | 135 ± 14 | 303 ± 99 | 2.4 | 5.4 | 0.0008 | <0.0001 |
Data are expressed as nanograms of MAb per gram of tissue.
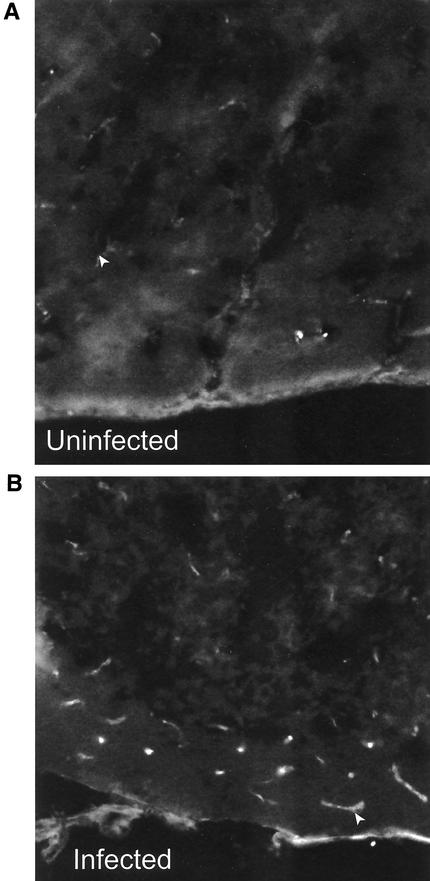
The dual radiolabeled MAb technique is a precise method for determining cell adhesion molecule levels, but it is possible that the increased P-selectin signal is due to increased vascular permeability, which increases the surface area for the binding antibody. To counter this possibility, the circulation time is kept short, minimizing diffusion. Indeed, ICAM-2 expression, which is constitutive, was similar in all the above organs during P. berghei malaria. To confirm that the changes in expression are due to increases in P-selectin on the brain endothelium, we performed immunofluorescence analysis on groups of three infected and three uninfected mice. The number of vessels labeling with P-selectin and the intensity of P-selectin fluorescence in the vessels increased markedly in brains of mice on day 6 of P. berghei infection compared with those found in uninfected controls (Fig. 3A and B).
FIG. 3.
Analysis of P-selectin levels in brain in uninfected C57BL/6 mice (A) and on day 6 of P. berghei infection (B) by fluorescence microscopy. Magnification (A and B), ×8.8. Arrow marks blood vessels in each section.
To ascertain whether the P-selectin expression in several organ beds is sufficient to mediate malarial pathogenesis, we compared P-selectin levels assessed by the dual radiolabeled MAb technique in two groups of five ECM-resistant BALB/c mice to the amount assessed in ECM-susceptible C57BL/6 controls. The first group of BALB/c mice was not infected, and the second was examined on day 6 of infection. Similar to the results obtained with ECM-susceptible C57BL/6 mice, ECM-resistant BALB/c mice demonstrated marked and significant (P < 0.05) increases in vascular P-selectin levels in brain and lung (5.5- and 7.0-fold with P = 0.003 and 0.004, respectively) as well as in gastrointestinal tract, heart, muscle, thymus, and pancreas (Table 2). A negative number was obtained for P-selectin in the kidneys of uninfected BALB/c mice, indicating that the binding of the nonspecific binding antibody was greater than that of the specific binding antibody. Nevertheless, marked vascular P-selectin was detected in the kidneys on day 6 of P. berghei infection, and the difference between infected and uninfected mice was significant (P = 0.0002). The parasitemia level was 2.5% ± 0.7% on day 4 and 26.8% ± 4.6% on day 6 of P. berghei infection (Fig. 2A), indicating that the BALB/c mice were adequately infected.
TABLE 2.
P-selectin in selected organs during the course of P. berghei malaria in groups of ECM-resistant BALB/c mice (n = 4 for all groups) (mean plus or minus standard deviation)
| Tissue | Level of P-selectina on
|
Change (n-fold) on day 6 vs day 0 | P for day 6 vs day 0 | |
|---|---|---|---|---|
| Day 0 | Day 6 | |||
| Heart | 9 ± 25 | 55 ± 6 | 6.0 | 0.01 |
| Pancreas | 32 ± 10 | 179 ± 29 | 5.5 | <0.0001 |
| Mesentery | 12 ± 4 | 134 ± 49 | 11.1 | 0.003 |
| Stomach | 44 ± 23 | 258 ± 89 | 5.9 | 0.004 |
| Small bowel | 6 ± 5 | 74 ± 15 | 13.3 | 0.0002 |
| Colon | 12 ± 14 | 100 ± 25 | 8.5 | 0.0002 |
| Cecum | 6 ± 11 | 68 ± 22 | 11.9 | 0.002 |
| Muscle | 36 ± 34 | 137 ± 15 | 3.8 | 0.002 |
| Thymus | 39 ± 31 | 117 ± 20 | 3.0 | 0.006 |
Data are expressed as nanograms of MAb per gram of tissue.
Leukocyte-endothelial cell interactions do not require P-selectin in brain during P. berghei malaria.
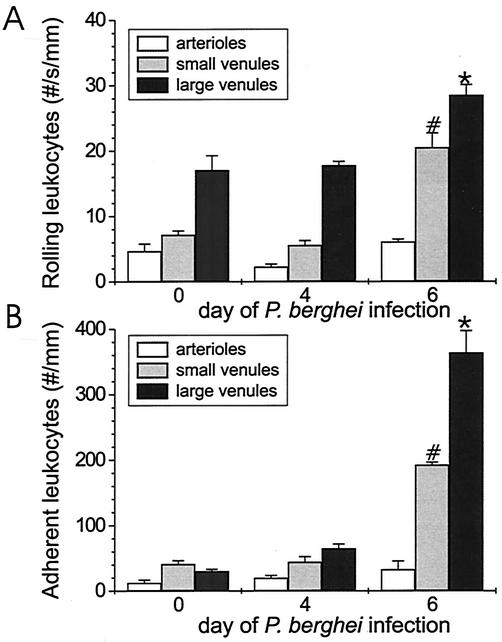
To define rolling and adhesion of leukocytes in vivo in brains of mice with cerebral malaria, leukocyte rolling and adhesion were assessed by intravital fluorescence microscopy in pial vessels of the brain microcirculatory system during the course of P. berghei malaria. Few rolling or adherent cells were observed in arterioles in either infected mice or uninfected controls (Fig. 4). Little rolling or adhesion of leukocytes was detected in brain venules of uninfected C57BL/6 mice. However, a marked and significant (P < 0.0001) increase in rolling and adhesion of leukocytes was observed in the brain venules of infected mice compared with those found in uninfected controls (3- and 2-fold for rolling leukocytes and 5- and 12-fold for adherent leukocytes in small and large postcapillary venules, respectively). The parasitemia level in the four mice per group was 3.0% ± 0.3% on day 4 and 11.4% ± 1.8% on day 6 of P. berghei infection, which indicates that the mice were appropriately infected.
FIG. 4.
Rolling (A) and adhesion (B) of leukocytes in brain microvasculature during the course of P. berghei malaria in ECM-susceptible C57BL/6 mice. Shown is the mean plus or minus SEM. At each time point, the brain circulation of five mice (five vessels) was assessed by intravital microscopy. A statistically significant (P < 0.05) difference in leukocyte rolling or adhesion in small (*) and large (#) postcapillary venules during the course of P. berghei infection was compared with leukocyte rolling and adhesion in uninfected C57BL/6 mice.
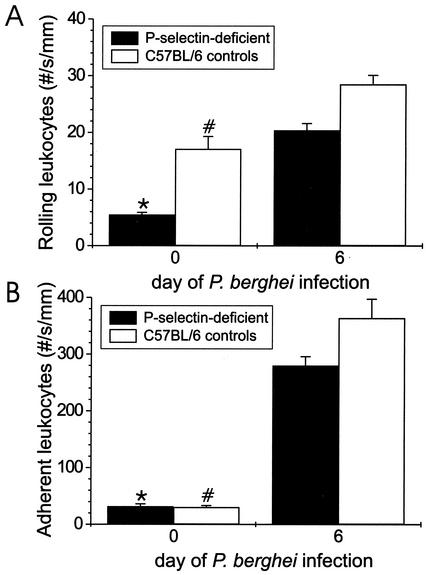
To determine the requirement of P-selectin for leukocyte rolling and adhesion on the endothelium in vivo and under flow during P. berghei malaria, we assessed rolling and adhesion of these cell types by using intravital microscopy in P-selectin-deficient mice infected with P. berghei. The parasitemia in the P-selectin-deficient mice was similar to that of P-selectin intact controls. The number of rolling leukocytes in uninfected P-selectin-deficient mice was lower than in uninfected C57BL/6 controls (P = 0.002), but the number of adherent leukocytes was similar in these two groups of mice (P = 0.2) (Fig. 5). The numbers of rolling and adherent leukocytes increased markedly (4- and 10-fold, respectively) and significantly (P < 0.0001) in P. berghei-infected P-selectin-deficient mice compared with those found in uninfected P-selectin-deficient mice. This change (n-fold) in leukocyte rolling and adhesion caused by P. berghei infection was similar in magnitude in the presence or absence of P-selectin. The number of rolling leukocytes was lower (P = 0.03) in P-selectin-deficient mice infected with P. berghei than in infected C57BL/6 controls. This decreased rolling did not alter adhesion, because the number of adherent leukocytes was similar (P = 0.2) in infected P-selectin-deficient mice and in infected C57BL/6 controls.
FIG. 5.
Rolling (A) and adhesion (B) of leukocytes to brain endothelium of mice lacking P-selectin on day 6 of P. berghei malaria. We assessed the mean plus or minus SEM of leukocyte rolling and adherence by using intravital microscopy in five brain venules of four infected and four uninfected P-selectin-deficient mice. The rolling and adherence of leukocytes in C57BL/6 controls on day 0 and day 6 of infection in panels A and B are repeated from Fig. 4 for comparison. Shown is a statistically significant (P < 0.05) difference in leukocyte rolling or adhesion from that found in P-selectin-deficient mice (*) or C57BL/6 controls (#) on day 6 of P. berghei infection (A and B).
DISCUSSION
Our observation that P-selectin-deficient mice are significantly (P = 0.005) protected from P. berghei malaria compared with C57BL/6 controls indicates that P-selectin functions in malarial pathogenesis. The major role for P-selectin is as a cell adhesion molecule mediating the rolling of leukocytes on the vascular endothelium during inflammation, and there is accumulating evidence of a strong inflammatory response during P. berghei malaria. High levels of proinflammatory cytokines (tumor necrosis factor and gamma interferon) are produced during P. berghei malaria, and these cytokines are required for malarial pathogenesis (12, 13, 27, 42). Moreover, production of anti-inflammatory interleukin 10 by resistant animals protects against the development of cerebral malaria (21). The proinflammatory cytokines in the blood may activate the endothelium to increase the expression of ICAM-1 (1, 26).
Infection with P. berghei markedly increases the endothelial P-selectin on brain microvasculature compared with that found in uninfected controls and increases the number of blood vessels labeled with P-selectin. The observation that the amount of ICAM-2, a constitutively expressed cell adhesion molecule, assessed by the dual radiolabeled MAb technique is not increased together with the confirmatory immunofluorescence studies indicates that the change in P-selectin protein occurs within the lumen of the blood vessel. We have observed by intravital microscopy platelets adhering to endothelial cells during P. berghei malaria. Thus, the increased P-selectin detected during malaria may be due to increased endothelial cell expression, activated platelet expression, or both. Our finding that P-selectin is markedly increased in several regional vascular beds supports the concept that ECM is a systemic inflammatory response. The increase of P-selectin in brain, lung, and kidneys suggests that P-selectin may contribute to the failure of these organs during ECM. Our observation that marked increases in P-selectin occur in several vascular beds in both ECM-susceptible (C57BL/6) and ECM-resistant (BALB/c) mice during P. berghei infection indicates that P-selectin expression by itself is not sufficient to mediate malarial pathogenesis.
Rolling and adhesion of leukocytes in the brain during inflammation (and by extension P. berghei malaria) are presently believed to be restricted to venules because of greater cell adhesion molecule expression in venules rather than differences in shear stress (2). This observation contrasts with the report that myelin basic protein-specific T cells adhere in brain arterioles, capillaries, and venules that are not inflamed to initiate the pathogenesis of experimental autoimmune encephalitis, an animal model of multiple sclerosis (39). Thus, the sites of adhesion in the brain are different under inflammatory conditions from those found under noninflammatory conditions.
Adhesion of leukocytes during P. berghei malaria to the activated endothelium is considered to be a requisite step in pathogenesis of experimental malaria, mediating the damage to the endothelium and subsequent tissue edema. Certainly, leukocytes are crucial for the development of severe P. berghei malaria, because few mice lacking polymorphonuclear leukocytes, CD4+ and CD8+ T cells, or macrophages develop severe malaria, whereas virtually all the controls succumb (15, 36, 42). The CD4+ and CD8+ T cells appear to contribute directly to endothelial damage because depletion of these cell types by MAb treatment during P. berghei malaria inhibits vascular leakage into brain assessed by Evans blue dye leakage and tissue edema measured by the wet/dry weight ratios (4).
P. berghei-infected mice exhibit marked leukocyte adhesion in the microvasculature compared with that found in uninfected controls, which is detectable by intravital microscopy, indicating that adherence is not an artifact. The greatest level of adherent leukocytes is on day 6 of infection, when the animals are moribund and when the greatest albumin leakage from the blood occurs into the brain, lung, heart, and kidney. Although P-selectin is required for leukocyte adherence to the microvasculature in several other inflammatory diseases (11), P-selectin is not required for leukocyte adherence during P. berghei malaria, because the numbers of adherent cells were similar in P-selectin-deficient mice and P-selectin-intact controls. It is possible that the cell adhesion molecules mediating leukocyte adherence are different in the brain from those in other tissues. Other candidate cell adhesion molecules are ICAM-1 and VCAM-1 (25, 28), which are adhesion molecules for P. falciparum (16).
Because P-selectin expression contributes to malarial pathogenesis but is not required for leukocyte adherence, P-selectin must have another important function. Studies of the human parasite P. falciparum indicate that P-selectin is an important adhesion molecule allowing parasitized erythrocytes to roll and adhere in vitro to endothelial cells under flow (17). Thus, P. berghei may use P-selectin to adhere to the endothelium, thereby obstructing blood flow in the brain and reducing the flow of oxygen and nutrients to the brain and impairing the removal of waste products from the brain. Indeed, lactic acidosis is a problem in both human and experimental malaria.
We speculate that another possibility, which is not mutually exclusive with the concept of P-selectin mediating parasite sequestration, is that P-selectin is important in mediating the adherence of platelets to the activated endothelium during malaria (30). Platelets are important components of the coagulation system and can induce endothelial damage. Inappropriate platelet activation during septic shock leads to disseminated intravascular coagulation. Disseminated intravascular coagulation is characterized by widespread blood clot formation, consuming coagulation factors and platelets, thereby rendering the patient predisposed to hemorrhage into several organs. The role of platelets in ECM is controversial, with depletion of platelets by MAb showing both protection and exacerbation of disease (24, 33). However, if platelet binding is inhibited by several different means, then protection is achieved (8). Our intravital studies confirm and extend these results by showing inhibition of platelet rolling and adhesion in P-selectin and ICAM-1-deficient mice infected with P. berghei (unpublished data). Thrombocytopenia occurs in both human and experimental malaria, and there are reports of shock, hemorrhaging, and disseminated intravascular coagulation in humans with severe malaria (22, 23).
Collectively, our results indicate that there is a marked upregulation of P-selectin in the brain and other organs during experimental malaria and that P-selectin contributes to the pathogenesis of malaria. Although there is marked leukocyte adhesion in the brains of P. berghei-infected mice, particularly when the animals are moribund, the adhesion of the leukocytes in the brain microvasculature does not require P-selectin.
Acknowledgments
This research was supported by NIH grants KO8 AI01438 (W.-L.C.), PO1 DK43785 (D.N.G.), and RO1 AI40667 (H.C.V.D.H.).
Editor: B. B. Finlay
REFERENCES
- 1.Amani, V., A. M. Vigario, E. Belnoue, M. Marussig, L. Fonseca, D. Mazier, and L. Renia. 2000. Involvement of IFN-gamma receptor-medicated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 30:1646-1655. [DOI] [PubMed] [Google Scholar]
- 2.Bienvenu, K., and D. N. Granger. 1993. Molecular determinants of shear rate-dependent leukocyte adhesion in postcapillary venules. Am. J. Physiol. 264:H1504-H1508. [DOI] [PubMed] [Google Scholar]
- 3.Campbell, J. J., J. Hedrick, A. Zlotnik, M. A. Siani, D. A. Thompson, and E. C. Butcher. 1998. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279:381-384. [DOI] [PubMed] [Google Scholar]
- 4.Chang, W.-L., S. P. Jones, D. J. Lefer, T. Welbourne, G. Sun, L. Yin, H. Suzuki, J. Huang, D. N. Granger, and H. C. van der Heyde. 2001. CD8+-T-cell depletion ameliorates circulatory shock in Plasmodium berghei-infected mice. Infect. Immun. 69:7341-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark, I. A., and L. Schofield. 2000. Pathogenesis of malaria. Parasitol. Today 16:451-454. [DOI] [PubMed] [Google Scholar]
- 6.Eling, W. M., and P. G. Kremsner. 1994. Cytokines in malaria, pathology and protection. Biotherapy 7:211-221. [DOI] [PubMed] [Google Scholar]
- 7.Falanga, P. B., and E. C. Butcher. 1991. Late treatment with anti-LFA-1 (CD11a) antibody prevents cerebral malaria in a mouse model. Eur. J. Immunol. 21:2259-2263. [DOI] [PubMed] [Google Scholar]
- 8.Favre, N., C. Da Laperousaz, B. Ryffel, N. A. Weiss, B. A. Imhof, W. Rudin, R. Lucas, and P. F. Piguet. 1999. Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes Infect. 1:961-968. [DOI] [PubMed] [Google Scholar]
- 9.Finley, R. W., L. J. Mackey, and P. H. Lambert. 1982. Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in cell-dependent nude mice. J. Immunol. 129:2213-2218. [PubMed] [Google Scholar]
- 10.Frenette, P. S., C. V. Denis, L. Weiss, K. Jurk, S. Subbarao, B. Kehrel, J. H. Hartwig, D. Vestweber, and D. D. Wagner. 2000. P-selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J. Exp. Med. 191:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granger, D. N., and P. Kubes. 1994. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J. Leukoc. Biol. 55:662-675. [PubMed] [Google Scholar]
- 12.Grau, G. E., L. F. Fajardo, P. F. Piguet, B. Allet, P. H. Lambert, and P. Vassalli. 1987. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210-1212. [DOI] [PubMed] [Google Scholar]
- 13.Grau, G. E., H. Heremans, P. F. Piguet, P. Pointaire, P. H. Lambert, A. Billiau, and P. Vassalli. 1989. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 86:5572-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hearn, J., N. Rayment, D. N. Landon, D. R. Katz, and J. B. de Souza. 2000. Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect. Immun. 68:5364-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermsen, C., T. van de Wiel, E. Mommers, R. Sauerwein, and W. Eling. 1997. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology 114:7-12. [DOI] [PubMed] [Google Scholar]
- 16.Ho, M., M. J. Hickey, A. G. Murray, G. Andonegui, and P. Kubes. 2000. Visualization of Plasmodium falciparum-endothelium interactions in human microvasculature: mimicry of leukocyte recruitment. J. Exp. Med. 192:1205-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho, M., T. Schollaardt, X. Niu, S. Looareesuwan, K. D. Patel, and P. Kubes. 1998. Characterization of Plasmodium falciparum-infected erythrocyte and P-selectin interaction under flow conditions. Blood 91:4803-4809. [PubMed] [Google Scholar]
- 18.Hoffmann, E. J., W. P. Weidanz, and C. A. Long. 1984. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect. Immun. 43:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaul, D. K., R. L. Nagel, J. F. Llena, and H. L. Shear. 1994. Cerebral malaria in mice: demonstration of cytoadherence of infected red blood cells and microrheologic correlates. Am. J. Trop. Med. Hyg. 50:512-521. [DOI] [PubMed] [Google Scholar]
- 20.Kawachi, S., S. Jennings, J. Panes, A. Cockrell, F. S. Laroux, L. Gray, M. Perry, H. van der Heyde, E. Balish, D. N. Granger, R. A. Specian, and M. B. Grisham. 2000. Cytokine and endothelial cell adhesion molecule expression in interleukin-10-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 278:G734-G743. [DOI] [PubMed] [Google Scholar]
- 21.Kossodo, S., C. Monso, P. Juillard, T. Velu, M. Goldman, and G. E. Grau. 1997. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 91:536-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishna, S., D. W. Waller, F. ter Kuile, D. Kwiatkowski, J. Crawley, C. F. Craddock, F. Nosten, D. Chapman, D. Brewster, and P. A. Holloway. 1994. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans. R. Soc. Trop. Med. Hyg. 88:67-73. [DOI] [PubMed] [Google Scholar]
- 23.Lagudis, S., L. F. Camargo, E. C. Meyer, C. J. Fernandes, N. Akamine, and E. Knobel. 2000. Hyperdynamic shock in falciparum malaria. Intensive Care Med. 26:142.. [DOI] [PubMed] [Google Scholar]
- 24.Lou, J., Y. R. Donati, P. Juillard, C. Giroud, C. Vesin, N. Mili, and G. E. Grau. 1997. Platelets play an important role in TNF-induced microvascular endothelial cell pathology. Am. J. Pathol. 151:1397-1405. [PMC free article] [PubMed] [Google Scholar]
- 25.Lou, J., R. Lucas, and G. E. Grau. 2001. Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clin. Microbiol. Rev. 14:810-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas, R., P. Juillard, E. Decoster, M. Redard, D. Burger, Y. Donati, C. Giroud, C. Monso-Hinard, T. De Kesel, W. A. Buurman, M. W. Moore, J. M. Dayer, W. Fiers, H. Bluethmann, and G. E. Grau. 1997. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur. J. Immunol. 27:1719-1725. [DOI] [PubMed] [Google Scholar]
- 27.Lucas, R., J. N. Lou, P. Juillard, M. Moore, H. Bluethmann, and G. E. Grau. 1997. Respective role of TNF receptors in the development of experimental cerebral malaria. J. Neuroimmunol. 72:143-148. [DOI] [PubMed] [Google Scholar]
- 28.Ma, N., N. H. Hunt, M. C. Madigan, and T. Chan-Ling. 1996. Correlation between enhanced vascular permeability, up-regulation of cellular adhesion molecules and monocyte adhesion to the endothelium in the retina during the development of fatal murine cerebral malaria. Am. J. Pathol. 149:1745-1762. [PMC free article] [PubMed] [Google Scholar]
- 29.Mackey, L. J., A. Hochmann, C. H. June, C. E. Contreras, and P. H. Lambert. 1980. Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin. Exp. Immunol. 42:412-420. [PMC free article] [PubMed] [Google Scholar]
- 30.Massberg, S., G. Enders, R. Leiderer, S. Eisenmenger, D. Vestweber, F. Krombach, and K. Messmer. 1998. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood 92:507-515. [PubMed] [Google Scholar]
- 31.Miller, L. H., M. F. Good, and G. Milon. 1994. Malaria pathogenesis. Science 264:1878-1883. [DOI] [PubMed] [Google Scholar]
- 32.Panes, J., M. A. Perry, D. C. Anderson, A. Manning, B. Leone, G. Cepinskas, C. L. Rosenbloom, M. Miyasaka, P. R. Kvietys, and D. N. Granger. 1995. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am. J. Physiol. 269:H1955-H1964. [DOI] [PubMed] [Google Scholar]
- 33.Polack, B., F. Delolme, and F. Peyron. 1997. Protective role of platelets in chronic (Balb/C) and acute (CBA/J) Plasmodium berghei murine malaria. Haemostasis 27:278-285. [DOI] [PubMed] [Google Scholar]
- 34.Rui-Mei, L., A. U. Kara, and R. Sinniah. 1998. In situ analysis of adhesion molecule expression in kidneys infected with murine malaria. J. Pathol. 185:219-225. [DOI] [PubMed] [Google Scholar]
- 35.Senaldi, G., C. L. Shaklee, J. Guo, L. Martin, T. Boone, W. Mak, and T. R. Ulich. 1999. Protection against the mortality associated with disease models mediated by TNF and IFN-gamma in mice lacking IFN regulatory factor-1. J. Immunol. 163:6820-6826. [PubMed] [Google Scholar]
- 36.Senaldi, G., C. Vesin, R. Chang, G. E. Grau, and P. F. Piguet. 1994. Role of polymorphonuclear neutrophil leukocytes and their integrin CD11a (LFA-1) in the pathogenesis of severe murine malaria. Infect. Immun. 62:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shear, H. L., M. W. Marino, C. Wanidworanun, J. W. Berman, and R. L. Nagel. 1998. Correlation of increased expression of intercellular adhesion molecule-1, but not high levels of tumor necrosis factor-alpha, with lethality of Plasmodium yoelii 17XL, a rodent model of cerebral malaria. Am. J. Trop. Med. Hyg. 59:852-858. [DOI] [PubMed] [Google Scholar]
- 38.Sinniah, R., L. Rui-Mei, and A. Kara. 1999. Up-regulation of cytokines in glomerulonephritis associated with murine malaria infection. Int. J. Exp. Pathol. 80:87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vajkoczy, P., M. Laschinger, and B. Engelhardt. 2001. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J. Clin. Investig. 108:557-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Andrian, U. H., and C. R. Mackay. 2000. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343:1020-1034. [DOI] [PubMed] [Google Scholar]
- 41.Wagner, D. D. 1993. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb. Haemostasis 70:105-110. [PubMed] [Google Scholar]
- 42.Yanez, D. M., D. D. Manning, A. J. Cooley, W. P. Weidanz, and H. C. van der Heyde. 1996. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J. Immunol. 157:1620-1624. [PubMed] [Google Scholar]