Abstract
The bacterial pathogen Helicobacter pylori is highly adapted to the human stomach, and a high level of polymorphism is observed among clinical isolates. This polymorphism may be the consequence of adaptive changes during colonization, making a strain better able to survive, to evade the immune system, and to provoke a chronic infection. To investigate the mechanisms involved in the acquisition of diversity in H. pylori, mouse models of single infections, coinfections, and superinfections were developed. These experimental infections were conducted with strain SS1, well known to be mouse adapted, and with two strains freshly isolated from infected patients: Hp141 and Hp145. Genetic modifications occurring in these strains were studied over time by comparing randomly selected colonies of the emerging strains to those of the infecting strains by using randomly amplified polymorphic DNA fingerprinting with six different primers and by using PCR to amplify the vacA and cagA genes. We showed that, regardless of the number of infecting strains, only one emerged from the animals and that the establishment of a first strain thwarted the implantation of a second strain. During both a single infection and a coinfection with SS1, Hp141 was replaced by a genetic variant (Hp141v) that overcame SS1 in coinfection experiments. Hp141v exhibited a deletion of a 102-bp repeated sequence within the ppk gene, which encodes polyphosphate kinase (PPK), an enzyme involved in the physiological adaptation of the microbial cell to nutritional and environmental stresses. The deletion led to higher enzymatic activity of PPK, and the variant exhibited a better capacity to colonize mice. Considering that the modified gene is known to be involved in adaptation to a new environment, our results are consistent with an adaptive change in strain Hp141 and suggest that PPK is an important virulence factor in H. pylori.
Helicobacter pylori is a gastrointestinal pathogen that colonizes the human stomach and is involved in chronic gastritis, peptic ulceration, and gastric carcinoma (25, 32, 40). Certain strains are able to infect animals, such as mice, which can be used as experimental infection models (13, 24). H. pylori is one of the bacteria that show the highest level of genetic polymorphism (1). The mechanisms leading to the diversity of H. pylori include mutation; intrachromosomal shift; variations in the locations of insertion sequences; mosaicism of genes, such as vacA; deletion of others, such as those included in the cag pathogenicity island; and recombination between strains coinfecting the same host (17, 23). The polymorphism of H. pylori could be the consequence of adaptive events during colonization of the human stomach, making a strain more efficient at evading the immune system and thus at surviving and provoking a long-term infection (29, 39).
In order to study the genetic changes in H. pylori that may occur during an infection, we developed a mouse model with strain SS1, previously adapted to this animal, and two strains freshly isolated from human patients (22). We assumed that the genetic modifications would be more pronounced and more easily detected if strains freshly isolated from one host species (human) were used to reinfect a different host species (mouse). The impact of the in vivo interactions between different infecting H. pylori strains was evaluated by comparing the results observed in single and multiple infections. We studied the evolution of the genome structures of these strains during experimental infections by comparing the infecting strains (IS) to the emerging strains (ES) isolated from the animals at different experimental steps. For that purpose, we used randomly amplified polymorphic DNA (RAPD) fingerprinting with six of the primers previously recommended by Akopyanz et al. (1) and PCR to amplify the genes encoding the two main virulence factors: vacA and cagA. The genetic changes detected were further characterized by sequencing of the involved genomic region, and the phenotypic consequences of the modifications were evaluated.
MATERIALS AND METHODS
H. pylori strains and growth conditions.
This study was conducted with three H. pylori strains: the Sydney strain (SS1), previously adapted to the mouse stomach and genetically stable (22), and two strains (Hp141 and Hp145) freshly isolated from patients hospitalized in Poitiers, France. Hp141 was isolated from a 32-year-old woman with gastritis, and Hp145 was isolated from a 36-year-old woman with a prepyloric ulcer.
To prepare the infecting inocula, a single colony of each of the three strains was cultured once on Skirrow medium (BioMerieux, Marcy l'Etoile, France) and then once in brucella broth for 2 days. For coinfection models, the inocula consisted of a mixture of two or three of these IS in brucella broth. Cultures were incubated at 37°C in a microaerobic atmosphere. Each strain was confirmed to belong to the species H. pylori by Gram staining and oxidase, catalase, and urease tests.
Experimental infections.
Experimental infections were conducted as previously described with 6-week-old female C57BL/6 inbred mice (13). Prior to the experimental infection, five randomly chosen animals were determined to be Helicobacter free by grinding their entire stomach and searching for H. pylori by (i) culturing on Skirrow medium and (ii) microscopic examination after Gram staining. These five animals were also checked for the absence of serum antibodies to H. pylori by an enzyme-linked immunosorbent assay (4). The animals were kept isolated from each other in cages stored within separate compartments ventilated with filtered air.
Single infections, coinfections, and superinfections were initiated at day 0. Inocula were administered intragastrically, twice at 2-h intervals, as 0.5-ml suspensions adjusted to 1010 CFU per ml. For single infections, 51 animals were infected at day 0 and individually sacrificed at day 3, 8, 15, 21, 45, 90, 150, or 365 postinoculation (p.i.) (Table 1). For coinfections, 10 animals were infected with a mixture of two or three IS (same amount of each) and sacrificed at day 45 p.i. For superinfections, a first IS was given at day 0 and another one was given at day 45 p.i.; the seven animals were sacrificed at day 90 p.i. As controls, 30 mice were given only brucella broth in order to check for the absence of contamination by human strains during the experiment, and 3 of them were sacrificed at each experimental step. The stomachs were entirely removed after sacrifice and ground in 1 ml of brucella broth. The suspensions were serially diluted and cultured on Skirrow medium for the determination of viable counts. The strains recovered were called ES. The numbers of viable H. pylori cells per stomach were determined after an incubation period of 7 days. To characterize the ES strains and to test for their genetic fingerprints, 15 randomly selected colonies were subcultured from culture-positive plates for each animal for RAPD analysis of 10 colonies (see below).
TABLE 1.
Results of experimental infections of C57BL/6 mice with H. pylori Hp141, Hp145, and SS1
| Infection model | Infecting strain(s) | Sacrifice interval (days) | No. of infected mice/no. tested | Colonization level (log CFU/stomach)a | ES profile |
|---|---|---|---|---|---|
| Single infection | Hp141 | 3 | 2/2 | 2-3.1 | Hp141 |
| 8 | 2/2 | 2.7-3.5 | Hp141 | ||
| 15 | 2/2 | 3.7-3.5 | Hp141 | ||
| 21 | 2/2 | 3.5-3.7 | Hp141 | ||
| 45 | 2/2 | 3.7-3.8 | Hp141 | ||
| 90 | 2/2 | 2.9-3.0 | Hp141 | ||
| 150 | 2/2 | 3.4-3.6 | Hp141 | ||
| 365 | 2/2 | 3.3-3.5 | Hp141 + Hp141v | ||
| Hp145 | 3 | 2/2 | 2.7-2.8 | Hp145 | |
| 8 | 2/2 | 2.8-3 | Hp145 | ||
| 15 | 2/2 | 3.1-3.4 | Hp145 | ||
| 21 | 2/2 | 3.6-3.7 | Hp145 | ||
| 45 | 2/2 | 3.6-3.8 | Hp145 | ||
| 90 | 2/2 | 3.5-3.8 | Hp145 | ||
| 150 | 2/2 | 3.1-3.3 | Hp145 | ||
| 365 | 2/2 | 3-3.5 | Hp145 | ||
| SS1 | 3 | 2/2 | 6-8.3 | SS1 | |
| 8 | 2/2 | 7.4-8 | SS1 | ||
| 15 | 2/2 | 6.1-8.3 | SS1 | ||
| 21 | 3/3 | 5.2-5.4-5.7 | SS1 | ||
| 45 | 3/3 | 4.3-4.5-4.6 | SS1 | ||
| 90 | 3/3 | 3.1-4.8-5.2 | SS1 | ||
| 150 | 2/2 | 2.3-3.8 | SS1 | ||
| 365 | 2/2 | 3.2-3.4 | SS1 | ||
| Coinfection | Hp141 + SS1 | 45 | 1/2 | 2.3 | SS1 |
| Hp145 + SS1 | 45 | 1/2 | 2.3 | SS1 | |
| Hp141 + Hp145 | 45 | 3/4 | 2.8-2.8-3.5 | Hp141 | |
| Hp141 + Hp145 + SS1 | 45 | 2/2 | 2.5 | Hp141v | |
| 2.3 | SS1 | ||||
| Superinfection | Hp141 and then Hp145 | 90 | 3/4 | 3.5-3.6-3.7 | Hp141 |
| Hp145 and then Hp141 | 90 | 2/3 | 1.6 | Hp145 | |
| 1.3 | Hp141 | ||||
| No infection (negative controls)b | None | 0/30 | 0 |
Values obtained for each infected mouse.
Three control mice were sacrificed at each interval.
In order to study competition between SS1 and IS Hp141 (wild type), ES Hp141 (a nonvariant strain isolated after 45 days in mice), or Hp141v (a variant strain emerging after 45 days in mice), coinfections were initiated at day 0 with a mixture containing the same amounts of SS1 and Hp141. The 15 animals (5 per mixture) were sacrificed at day 15 p.i. The ES strains were characterized as described above.
The number of bacteria necessary to infect 50% of the animals (ID50) was determined for IS Hp141, ES Hp141, or Hp141v by using bacterial suspensions adjusted to 1010, 108, 106, and 104 CFU per ml. The 60 animals (5 per inoculum) were sacrificed at day 15 p.i. The number of viable H. pylori cells was determined as described above.
These experimental infection protocols received the approval of the Ethics Committee of the University of Poitiers (number MIC/2001/07/AC).
RAPD analysis.
Genomic DNAs of the IS and the ES were extracted with purification columns (QIAmp DNA minikit) according to the protocol described by the manufacturer (Qiagen, Courtaboeuf, France). Six primers were chosen from those described by Akopyanz et al. (1): 1254, 1283, 1247, 1281, 1290, and D9355. PCR amplification was performed with a 50-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 6 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 50 pM each primer, 1.25 U of Taq polymerase (Amersham-Pharmacia-Biotech, Orsay, France), and 40 ng of template DNA. In total, 30 colonies of IS and 630 colonies of ES were analyzed by RAPD analysis.
Amplification was performed with a Perkin-Elmer (Courtaboeuf, France) 9600 thermal cycler. The cycling program for the six primers was as follows: 4 cycles of 94°C for 5 min, 36°C for 5 min, and 72°C for 5 min; 35 cycles of 94°C for 1 min, 36°C for 1 min, and 72°C for 2 min; and then 72°C for 10 min. RAPD products were analyzed by electrophoresis in 2% agarose gels, stained with ethidium bromide, and then visualized with UV light. DNA marker VI (Roche Molecular Biochemicals, Meylan, France) was used as a size marker in all gels. All DNA extractions and RAPD analysis were repeated three times to ensure reproducibility.
Cloning and sequencing.
DNA from the additional 950-bp fragment that appeared in the Hp141v RAPD profile was eluted from agarose by using a Wizard PCR Preps DNA purification system (Promega, Madison, Wis.). After PCR amplification with primer 1254, the fragment was cloned by using a TOPO TA cloning kit for sequencing and One Shot TOP 10 chemically competent Escherichia coli (Invitrogen, Cergy-Pontoise, France). Plasmid DNA was extracted by using a QIA Prep Spin miniprep kit (Qiagen) and PCR amplified by using the T3 and T7 primers included in the cloning kit. Sequencing of the insert was carried out with an ABI Prism DNA sequencing kit and an ABI Prism 310 genetic analyzer (Applied Biosystems, Warrington, United Kingdom). Sequence analysis was carried out with Sequencing Analysis and Sequence Navigator software (Applied Biosystems). The obtained genomic sequence was compared with the genomic sequences of H. pylori 26695 and J99 in the genome databases of The Institute for Genomic Research (www.tigr.org) (2, 42).
PCR amplifications. (i) vacA and cagA genes.
Genomic DNAs of the IS and the ES were extracted by using the same method as that used for RAPD analysis. The cagA status of the H. pylori strains was determined with primers and PCR conditions previously described by Tummuru et al. (43). For typing of the vacA gene, fragments m1, m2, s1a, s1b, and s2 were PCR amplified with primers and PCR conditions recommended by Atherton et al. (3).
(ii) ppk gene.
Five sets of primers were chosen on the basis of the H. pylori 26695 and J99 ppk genetic sequences (Table 2). PCR amplification was performed with a 50-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 3 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 50 pM each primer, 1.25 U of Taq polymerase (Roche), and 40 ng of DNA extracted as described above. The cycling conditions consisted of an initial denaturation at 94°C for 7 min followed by 35 cycles of 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min and then by a final elongation at 72°C for 7 min.
TABLE 2.
Primers used for amplification of the ppk gene
| Primer | Sequence | Amplified fragment |
|---|---|---|
| ppk1 | 5-TTGAATCGTTTCTTTAACCGA-3 | ppkA |
| ppk2 | 5-TGATGTGGGCAAAAAGCGCGA-3 | |
| ppk3 | 5-CTTCGCACACCTTCCGCGCTT-3 | ppkB |
| ppk4 | 5-CCCCTAAATTGAGCATGATCGCGC-3 | |
| ppk5 | 5-CTCACCGGCATGCATATCTAT-3 | ppkC |
| ppk6 | 5-AGTGATCACGAGCATTTTAGC-3 | |
| ppk7 | 5-TAGAAAGGGCGGGCGCGTTAG-3 | ppkD |
| ppk8 | 5-GCTCAAGCCCTTGACTTGGG-3 | |
| ppk9 | 5-GAATGGCTCTATCAAGCCTCT-3 | ppkE |
| ppk10 | 5-AAGGGTTTTAAGGGCTTGTTT-3 |
PolyP extraction and determination.
Total intracellular polyphosphate (PolyP) was assayed by the method of McGrath and Quinn (27). Cells of IS Hp141, ES Hp141, and Hp141v were grown in brucella broth complemented with 5% fetal calf serum. The bacterial cells were harvested by centrifugation at 10,000 × g for 15 min at 4°C and washed twice in 1.5 M NaCl containing 0.01 M EDTA and 1 mM NaF (washing buffer). The cell pellet was resuspended in 1.5 ml of washing buffer and sonicated on ice for 10 30-s periods with 2-min intervals at 16 kHz. The resulting homogenate was centrifuged at 25,000 × g for 60 s at 4°C to remove cell debris. To determine total intracellular PolyP, 200 μl of concentrated HCl was added to 1 ml of cell extract, and the mixture was heated at 100°C for 45 min; the PolyP liberated was assayed by the method of Kyaw et al. (21). The concentrations were expressed in micromoles of phosphate per milligram of cellular protein and are given as means of triplicates. An unhydrolyzed sample was used as a control to determine the background level of inorganic phosphate (Pi). Protein concentrations were determined with a bicinchoninic acid protein assay reagent kit (Pierce Chemicals, Rockford, Ill.). Growth was measured by the determination of viable H. pylori cells after plating of 50 μl of a serially diluted culture on Skirrow medium.
RESULTS
Characterization of H. pylori IS.
C57BL/6 mice were experimentally inoculated with one, two, or three H. pylori strains. These three strains were distinguishable from each other by their RAPD patterns obtained with six distinct primers, their cagA status, and their vacA genotype. SS1 was cagA positive and of the m2s2 vacA genotype, Hp141 was cagA positive and of the m1s1a vacA genotype, and Hp145 was cagA negative and of the m2s2 vacA genotype. The infection was successful with all three strains.
Analysis of experimental infections (Table 1).
Ten randomly selected colonies obtained from each animal were studied independently by RAPD analysis and PCR of the cagA and vacA genes. RAPD analysis showed that each mouse was colonized by a unique strain regardless of the inoculation model. The cagA status and the vacA genotype of the different ES were the same as those of the IS.
In single infections, all 51 mice were successfully infected. H. pylori was recovered at each experimental step during the 1-year follow-up. Nevertheless, differences in colonization levels were found between SS1 and the two clinical strains, Hp141 and Hp145. The latter two strains led to similar colonization levels, which varied between 102 and 104 CFU per stomach, with a maximum at day 45 p.i. The colonization levels obtained with SS1 reached 107 CFU per stomach during the first 2 weeks of infection and then decreased to reach the values observed with the other two strains at day 150 p.i. All of the ES had the same RAPD pattern as the IS, except at day 365 p.i. for the two mice inoculated with Hp141: 16 out of the 20 emerging colonies had the RAPD profile of IS Hp141 with five out of the six primers. However, with primer 1254, an additional 950-bp DNA fragment appeared. This modified ES was called Hp141v.
In coinfections, animals were inoculated with a mixture of two or three H. pylori strains. After 45 days, H. pylori was recovered from the stomachs of 7 out of the 10 animals, and the colonization levels in the infected mice were found to be 102 to 103 CFU per stomach. We never detected Hp145. Each time that SS1 was present in the infecting inoculum, it was recovered as an ES, except in one mouse having received the three H. pylori strains. In this case, the 10 emerging colonies had the same RAPD profile. This profile was identical to the Hp141v profile (Fig. 1).
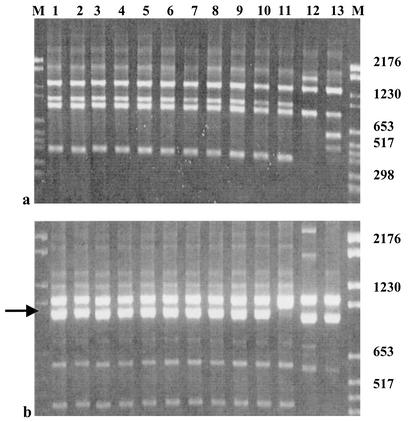
FIG. 1.
RAPD patterns obtained with primers 1290 (a) and 1254 (b). Lanes 1 to 10, 10 colonies emerging from a mouse coinfected with Hp141, Hp145, and SS1; lane 11, IS Hp141; lane 12, IS Hp145; lane 13, IS SS1; lanes M, molecular weight markers. Profiles obtained with primer 1290 for the 10 emerging colonies are identical to those of IS Hp141; similar results were obtained with primers 1283, 1247, 1281, and D9355. Profiles obtained with primer 1254 showed that the 10 studied colonies had an additional 950-bp DNA fragment (black arrow) compared to IS Hp141. Numbers at right indicate base pairs.
In superinfections, mice received first Hp141 or Hp145 and then received, at day 45, the other strain. At day 90 p.i., H. pylori was recovered from five out of the seven infected animals, and the colonization levels varied according to the order of inoculation of the strains. Mice inoculated with Hp141 first had a mean colonization level of 4 × 103 CFU per stomach; that for mice inoculated with Hp145 first was only 30 CFU per stomach. Hp141 was recovered from all of the animals except one, which received Hp145 first. No genetic modification of the strains was detected in this model.
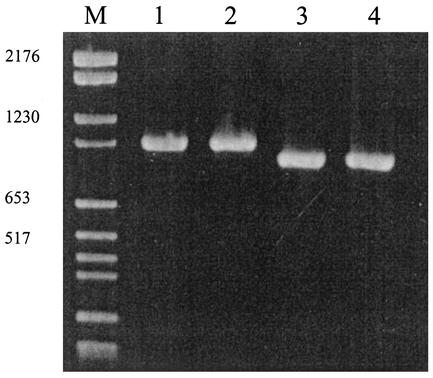
The presence of the variant in low quantities in the infecting inocula was checked a posteriori by PCR with primers ppk1 and ppk4. Within a 48-h culture of IS Hp141 initially given to the animals, we detected only one PCR fragment corresponding to wild-type polyphosphate kinase (PPK) (Fig. 2).
FIG. 2.
Search for Hp141v in the Hp141 infecting inoculum by PCR with primers ppk1 and ppk4. Lanes 1 and 2, two different IS Hp141 DNA extracts; lanes 3 and 4, two different Hp141v DNA extracts; lane M, molecular weight markers. No Hp141v DNA was detected in IS Hp141 by this method. Numbers at left indicate base pairs.
Characterization of Hp141v.
Sequencing of the additional 950-bp DNA fragment observed in strain Hp141v revealed a modification in the PPK gene (ppk), referenced as open reading frame number HP1010 from H. pylori 26695 or JHP0413 from H. pylori J99 (95% identity with Hp26695 and 97% identity with J99). On the basis of the ppk sequence of strain J99, five sets of primers were chosen in order to amplify and sequence the entire ppk gene in IS Hp141 and Hp141v. A comparison of the two sequences revealed a deletion of a 102-bp repeated sequence between nucleotides 449 and 551 in the variant ppk gene (Fig. 3).
FIG. 3.
Genomic sequence of the ppkA fragment amplified in IS Hp141 (I) and Hp141v (V) with primers ppk1 and ppk2. The difference between the two strains consisted of a deletion of a 102-bp repeated sequence in the Hp141v gene (in bold characters). No other modification was detected in the entire sequenced gene.
PolyP levels.
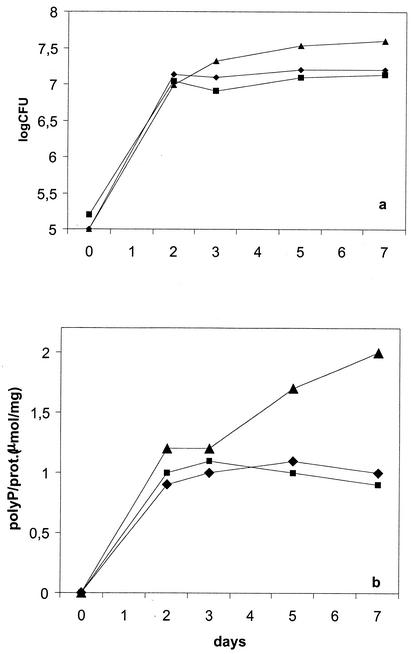
Total intracellular PolyP levels were determined during the growth of IS Hp141, ES Hp141, and Hp141v. As shown in Fig. 4, PolyP levels were similar for IS Hp141 and ES Hp141. However, the growth of Hp141v was associated with a significant accumulation of PolyP and a better growth yield in the stationary phase.
FIG. 4.
Growth (a) and PolyP levels (b) monitored over 7 days for IS Hp141 (⧫), ES Hp141 (▪), and Hp141v (▴). Each point represents the mean of three determinations. prot., protein.
Differences in the infectivities of IS Hp141, ES Hp141, and Hp141v.
Infectivities were first estimated by determination of the ID50s for the studied strains. For each strain, bacterial inocula containing 1010, 108, 106, and 104 CFU/ml were inoculated into four groups of mice. The log ID50s determined graphically were 9.3, 7.8, and 6.4 for IS Hp141, ES Hp141, and Hp141v, respectively. Infectivities were also determined by in vivo competition between each one of the three Hp141 strains and the mouse-adapted strain, SS1. With the methods used, we detected 100, 80, and only 23% SS1 in mice inoculated with mixtures containing IS Hp141, ES Hp141, and Hp141v, respectively. These proportions were found to be significantly different by the chi-square test (P < 0.001).
DISCUSSION
H. pylori has been described as one of the most polymorphic bacterial species. This polymorphism may be the consequence of mutations, horizontal uptake of homologous or heterologous DNA, or genetic shifts (10, 16, 26; B. Björkholm, L. Engstrand, and P. Falk, Proc. 3rd Int.Workshop Pathogenesis Host Response Helicobacter Infect., abstr. no. J1, 1998). Because the stomach of infected mammals is the unique niche of this bacterial species, the genetic events leading to H. pylori polymorphism probably occur during the infection and may contribute to the adaptation process allowing for the chronicity of the infection. In this work, we searched for genetic modifications during experimental infections by using the RAPD fingerprint method, which allows for the evaluation of genetic polymorphisms over the entire genome and which is universally recognized for its excellent discriminatory power (1). We also used PCR amplification of the cagA and vacA genes, well known to have variable genetic sequences (6, 9, 14).
The experimental infections were carried out with C57BL/6 mice, preceding studies having shown that this mouse strain has the best characteristics for both susceptibility to colonization and persistence of infection (22, 36; X. Wang, R. Willen, T. Wadstrom, and P. Aleljung, Proc. 3rd Int.Workshop Pathogenesis Host Response Helicobacter Infect., abstr. no. J8, 1998). The IS were either mouse adapted (SS1) or freshly isolated from a human host. We expected more adaptive modifications during the mouse infection when the two clinical strains considered to be human adapted were used.
A 1-year follow-up of the single infections allowed us to establish the timing of the events occurring during a long-term infection. Analysis of single infections, coinfections, and superinfections provided interesting data regarding the ability of a strain to colonize the gastric mucosa and the ecologic rules governing the establishment of a strain at the expense of another strain. Regardless of the infection model, we did not detect, among the 10 studied colonies of the strain emerging from each animal and subjected to RAPD analysis, the simultaneous presence of different strains in the stomach of a given animal. Colonization levels were lower in multiple infections than in single infections. In multiple infections carried out with the clinical strains, Hp145 was detected only once, in a superinfection model when it was given first. Thus, Hp141 seems to be more mouse adapted than Hp145, and the order of inoculation of the strains seems important. Once established, a strain could interfere with the establishment of a subsequent strain even when the latter was more fit to colonize mice. This phenomenon was previously described by Danon et al., who showed that the implantation of a first strain in the mouse could prevent the implantation of a mixture of other strains (7). This finding could be the consequence of the immune response against the first strain (IS) being strong enough to inhibit the implantation of the second strain. It could also be due to competition between strains for nutritional factors and/or to differences in the virulence of the inoculated strains. In this work, Hp141 was cagA positive and produced an active vacuolizing cytotoxin, while Hp145 did not. Despite the fact that the roles of these factors in the extent of colonization are quite controversial, it should be noted that the more virulent strain (Hp141) was also better able to colonize mice. In some studies, the presence of the two markers was considered essential for the success of mouse colonization (6, 33), while in other studies, colonization ability did not depend on the cagA status or the vacA genotype of the strains (5, 17, 35, 37; L. J. Thompson, S. Danon, J. Wilson, J. O'Rourke, N. Salama, S. Falkow, H. Mitchell, and A. Lee, Int. J. Med. Microbiol. 291:137, abstr. O.25, 2001). Other studies showed that mice or rhesus monkeys infected with mixtures of H. pylori strains were only transiently infected by more than one strain. However, they became persistently infected by only one strain for weeks or months (7, 8, 37; Wang et al., Proc. 3rd Int.Workshop Pathogenesis Host Response Helicobacter Infect., abstr. no. J8). Nevertheless, polycolonizations in humans have been reported (11, 15, 38, 44). This difference between humans and animals may be due to the major susceptibility of humans, the natural host of H. pylori, but may also be due to differences between H. pylori strains.
This is the first report of the occurrence of genetic modifications during both a single infection and multiple infections, indicating that they do not result from genetic exchanges between H. pylori strains. The variant was not detected by PCR in the infecting inoculum and was isolated during the last steps of the single infection. This result suggests that the modified strain appeared during the infection or was present in the infecting inoculum but was below the limit of detectability of the method used and was selected for by the mouse environment. Moreover, the selection of Hp141v two times independently strongly suggests that the genetic modifications lead to an advantage for the colonization of mice.
The occurrence of genetic modifications during a single infection is rare. In the study of Bjorkholm et al. (6), colonization experiments in animals were simply too short for any discernible genetic changes to occur. Interestingly, in our work, the emerging variant appeared after one full year of infection. Several years of follow-up of strains from infected patients with common genotyping methods, such as RAPD analysis, pulsed-field gel electrophoresis, and PCR-restriction fragment length polymorphism analysis, revealed no modifications. Only whole-genome DNA microarray analysis or sequencing revealed the emergence of subclones with micromodifications (6, 12, 34). These data suggest that the diversity of H. pylori may be due in part to continuous microevolution that would be detectable only after a long-term infection.
Analysis of Hp141v allowed us to identify a genetic modification consisting of a 102-bp deletion in the gene encoding PPK. This enzyme reversibly catalyzes the transfer of the gamma phosphate of ATP to PolyP (18). Several studies based on disruption of the ppk gene have shown that PolyP levels are directly proportional to PPK activity (30, 41). In order to study the consequences of the observed deletion for PPK activity, we determined PolyP levels in IS Hp141, ES Hp141, and Hp141v. Our data are consistent with better enzymatic activity of Hp141v, since PolyP levels in this strain were considerably higher than those in wild-type Hp141 and ES Hp141.
The results of the coinfections suggested that Hp141v should have better colonization properties than the wild-type strain, since it overcame the mouse-adapted strain, SS1. This point was confirmed by additional competition experiments with SS1 and IS Hp141, ES Hp141, or Hp141v. It can be expected that after some additional weeks of experimental competition, only one strain will emerge, as in the 45-day coinfections, and that SS1 will disappear from the isolates recovered from the mice inoculated with Hp141v.
The ability of Hp141v to compete with other strains for mouse colonization is notably better than that of the wild-type strain or a nonvariant ES. Moreover, Hp141v seems irreversibly better adapted to mice than SS1. The infectivity of Hp141v was also compared with the infectivities of IS Hp141 and ES Hp141 by determination of the ID50s. The important difference between the ID50s of Hp141v and the other two strains is consistent with the better ability of Hp141v to colonize mice. These data strongly suggest that the ppk gene is crucial for the virulence of H. pylori in the colonization of mice. However, the construction of an isogenic mutant by disruption of the ppk gene in Hp141v and testing of its ability to colonize mice would be of great interest for excluding the implication of other mutations not detected by the methods used and for confirming the role of PPK in this phenomenon.
It is now well known that PPK is active in numerous and various bacterial mechanisms designed for the reservation of energy and phosphate; chelation of divalent cations; resistance to the action of complement; serving as a component of capsules; buffering against pH variations; “channeling” for DNA entry during bacterial transformation; motility; quorum sensing of bacterial pathogens; inhibition of RNA degradation; and resistance to heat, oxidants, osmotic shock, and amino acid starvation by promoting ribosomal degradation during the stationary phase of growth (18, 19, 20, 28, 30, 31, 41). PPK may therefore play important roles in the physiological adaptation of microbial cells during growth and development and in their responses to nutritional and environmental stresses. In this respect, our results are consistent with an adaptive change of Hp141 and imply that, like those of other bacterial pathogens, the PPK of H. pylori is an essential virulence factor that could be used as a target for antimicrobial drugs (19, 20, 31).
In conclusion, we described several experimental mouse infections and reported genetic events that might improve the adaptation of strains to the current host. Thus, genetic modifications occurring during the course of an H. pylori infection may be involved in the acquisition of the genetic polymorphism of this bacterial species.
Acknowledgments
We thank Laboratoire de Microbiologie B, CHU La Milétrie, Poitiers, France, for technical assistance in DNA sequencing and Joyce Johnson for help in improving the English in the manuscript.
We are grateful to the Ligue Contre le Cancer, Comité de Charente Maritime, and to Université de Poitiers and CHU La Milétrie, Poitiers, France, for financial support.
Editor: V. J. DiRita
REFERENCES
- 1.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 20:5137-5142.U. K. [DOI] [PMC free article] [PubMed]
- 2.Alm, R. A., L. S. L. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. Dejonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nackelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 3.Atherton, J. C., P. Cao, R. M. Peek, M. K. Tummuru, Jr., M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 4.Aucher, P., M. L. Petit, P. R. Mannant, L. Pezennec, P. Babin, and J. L. Fauchère. 1998. Use of immunoblot assay to define serum antibody patterns associated with Helicobacter pylori infection and with H. pylori-related ulcers. J. Clin. Microbiol. 36:931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayraud, S., B. Janvier, and J. L. Fauchere. 2002. Experimental colonization of mice by fresh clinical isolates of Helicobacter pylori is not influenced by the cagA status and the vacA genotype. FEMS Immunol. Med. Microbiol. 34:169-172. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkholm, B., A. Lundin, A. Sillen, K. Guillemin, N. Salama, C. Rubio, J. I. Gordon, P. Falk, and L. Engstrand. 2001. Comparison of genetic divergence and fitness between two subclones of Helicobacter pylori. Infect. Immun. 69:7832-7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danon, S. J., B. J. Luria, R. E. Mankoski, and K. A. Eaton. 1998. RFLP and RAPD analysis of in vivo genetic interactions between strains of Helicobacter pylori. Helicobacter 3:254-259. [DOI] [PubMed] [Google Scholar]
- 8.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, G. J. Perez-Perez, and M. J. Blaser. 1996. Transient and persistent experimental infections of nonhuman primates with Helicobacter pylori: implications for human disease. Infect. Immun. 64:2885-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garner, J. A., and T. L. Cover. 1995. Analysis of genetic diversity in cytotoxin-producing and non-cytotoxin-producing Helicobacter pylori strains. J. Infect. Dis. 172:290-293. [DOI] [PubMed] [Google Scholar]
- 10.Go, M. F., V. Kapur, D. Y. Graham, and J. M. Musser. 1996. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J. Bacteriol. 27:1870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirschl, A. M., M. Richter, A. Makristathis, P. M. Pruckl, B. Willinger, K. Scutze, and M. L. Rotter. 1994. Single and multiple strain colonization in patients with Helicobacter pylori-associated gastritis: detection by macrorestriction DNA analysis. J. Infect. Dis. 170:473-475. [DOI] [PubMed] [Google Scholar]
- 12.Israel, D. A., N. Salama, U. Krishna, U. M. Rieger, J. C. Atherton, S. Falkow, and R. M. Peek. 2001. Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc. Natl. Acad. Sci. USA 98:14625-14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janvier, B., B. Grignon, C. Audibert, L. Pezennec, and J. L. Fauchere. 1999. Phenotypic changes of Helicobacter pylori components during an experimental infection in mice. FEMS Immunol. Med. Microbiol. 24:27-33. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, Q., K. Hiratsuka, and D. E. Taylor. 1996. Variability of gene order in different Helicobacter pylori strains contributes to genome diversity. Mol. Microbiol. 20:833-842. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen, M., G. Daskalopoulos, V. Warburton, H. M. Mitchell, and S. L. Hazell. 1996. Multiple strain colonization and metronidazole resistance in Helicobacter pylori-infected patients: identification from sequential and multiple biopsy specimens. J. Infect. Dis. 174:631-635. [DOI] [PubMed] [Google Scholar]
- 16.Kersulyte, D., H. Chalkauskas, and D. E. Berg. 1999. Emergence of recombinant strains of Helicobacter pylori during human infection. Mol. Microbiol. 31:31-43. [DOI] [PubMed] [Google Scholar]
- 17.Konturek, P. C., T. Brzozowski, S. J. Konturek, J. Stachura, E. Karczewska, R. Pajdo, P. Ghiara, and E. G. Hahn. 1999. Mouse model of Helicobacter pylori infection: studies of gastric function and ulcer healing. Aliment. Pharmacol. Ther. 13:333-346. [DOI] [PubMed] [Google Scholar]
- 18.Kornberg, A. 1995. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J. Bacteriol. 177:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornberg, A., N. N. Rao, and D. Ault-Riche. 1999. Inorganic polyphosphate: a molecule of many functions. Annu. Rev. Biochem. 68:89-125. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda, A., K. Nomura, R. Ohtomo, J. Kato, T. Ikeda, N. Takiguchi, H. Ohtake, and A. Kornberg. 2001. Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 293:705-712. [DOI] [PubMed] [Google Scholar]
- 21.Kyaw, A., K. Maung-U, and T. Toe. 1985. Determination of inorganic phosphate with molybdate and triton X-100 without reduction. Anal. Biochem. 145:230-234. [DOI] [PubMed] [Google Scholar]
- 22.Lee, A., J. O'Rourke, M. Corazon de Ungria, B. Robertson, G. Daskapopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 23.Marais, A., G. L. Mendz, S. L. Hazell, and F. Megraud. 1999. Metabolism and genetics of Helicobacter pylori: the genome era. Microbiol. Mol. Biol. Rev. 63:642-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 25.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed]
- 26.Maynard Smith, J., and N. H. Smith. 1998. Detecting recombination from gene trees. Mol. Biol. Evol. 15:590-599. [DOI] [PubMed] [Google Scholar]
- 27.McGrath, J. W., and J. P. Quinn. 2000. Intracellular accumulation of polyphosphate by the yeast Candida humicola G-1 in response to acid pH. Appl. Environ. Microbiol. 66:4068-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullan, A., J. P. Quinn, and J. W. McGrath. 2002. Enhanced phosphate uptake and polyphosphate accumulation in Burkholderia cepacia grown under low pH conditions. Microb. Ecol. 44:69-77. [DOI] [PubMed] [Google Scholar]
- 29.Oudbier, J. H., W. Langenberg, E. A. J. Rauws, and C. Bruin-Mosch. 1990. Genotypical variation of Campylobacter pylori from gastric mucosa. J. Clin. Microbiol. 28:83-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashid, M. H., N. N. Rao, and A. Kornberg. 2000. Inorganic polyphosphate is required for motility of bacterial pathogens. J. Bacteriol. 182:225-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashid, M. H., K. Rumbaugh, L. Passador, D. G. Davies, A. N. Hamood, B. Iglewski, and A. Kornberg. 2000. Polyphosphate kinase is essential for biofilm development, quorum sensing and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:9636-9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rautelin, H., and T. U. Kosunen. 1991. Helicobacter pylori and associated gastroduodenal diseases. APMIS 99:677-695. [PubMed] [Google Scholar]
- 33.Salama, N. R., G. Otto, L. Tompkins, and S. Falkow. 2001. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 69:730-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salama, S. M., Q. Jiang, N. Chang, R. W. Sherbaniuk, and D. E. Taylor. 1995. Characterization of chromosomal DNA profiles from Helicobacter pylori strains isolated from sequential gastric biopsy specimens. J. Clin. Microbiol. 33:2496-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheu, B. S., H. B. Yang, J. J. Wu, A. H. Huang, X. Z. Lin, and I. J. Su. 1999. Development of Helicobacter pylori infection model in BALB/c mice with domestic cagA-positive and -negative strains in Taiwan. Dig. Dis. Sci. 44:868-875. [DOI] [PubMed] [Google Scholar]
- 36.Smythies, L. E., J. A. Chen, J. R. Lindsey, P. Ghiara, P. D. Smith, and K. B. Waites. 2000. Quantitative analysis of Helicobacter pylori infection in a mouse model. J. Immunol. Methods 242:67-78. [DOI] [PubMed] [Google Scholar]
- 37.Sturegard, E., H. Sjunnesson, H. Nilsson, R. Andersson, C. Areskoug, and T. Wadstrom. 2001. Infection with cagA- and vacA-positive and -negative strains of Helicobacter pylori in a mouse model. FEMS Immunol. Med. Microbiol. 30:115-120. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, N. S., J. G. Fox, N. S. Akopyants, D. E. Berg, N. Thompson, B. Shames, L. Yan, E. Fontham, F. Janney, F. M. Hunter, et al. 1995. Long-term colonization with single and multiple strains of Helicobacter pylori assessed by DNA fingerprinting. J. Clin. Microbiol. 33:918-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tee, W., J. Lambert, R. Smallwood, M. Schembri, B. C. Ross, and B. Dwyer. 1992. Ribotyping of Helicobacter pylori from clinical specimens. J. Clin. Microbiol. 30:739-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The EUROGAST Study Group. 1993. An international association between Helicobacter pylori infection and gastric cancer. Lancet 341:1359-1362. [PubMed] [Google Scholar]
- 41.Tinsley, C. R., and E. Gotschlich. 1995. Cloning and characterization of the meningococcal polyphosphate kinase gene: production of polyphosphate synthesis mutants. Infect. Immun. 63:1624-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleishmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodeck, K. McKenney, L. M. Fitzerald, N. Lee, M. D. Adams, D. E. Berg, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 43.Tummuru, M., T. L. Cover, and M. J. Blaser. 1993. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of a linkage to cytotoxin production. Infect. Immun. 61:1799-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yakoob, J., X. G. Fan, G. L. Hu, H. X. Yang, S. H. Liu, D. M. Tan, T. G. Li, and Z. Zhang. 2001. Polycolonization of Helicobacter pylori among chinese subjects. J. Clin. Microbiol. Infect. 7:187-192. [DOI] [PubMed] [Google Scholar]